
Am Fam Physician. 1998;57(2):279-286
See related patient information handout on insulin lispro.
Research has established the importance of maintaining blood glucose levels near normal in patients with type 1 (insulin-dependent) diabetes mellitus. Short-acting insulin analogs are designed to overcome the limitations of regular short-acting insulins. Compared with regular human insulin, the analog insulin lispro offers faster subcutaneous absorption, an earlier and greater insulin peak and a more rapid post-peak decrease. Insulin lispro begins to exert its effects within 15 minutes of subcutaneous administration, and peak levels occur 30 to 90 minutes after administration. Duration of activity is less than five hours. Rates of insulin allergy, lipodystrophy, hypoglycemia and abnormal laboratory test results are essentially the same in patients using insulin lispro and in those using regular human insulin.
The Diabetes Control and Complications Trial (DCCT)1 established the importance of maintaining near-normal blood glucose levels in patients with type 1 (insulin-dependent) diabetes mellitus. In these patients, intensive therapeutic regimens have been found to delay the onset and reduce the progression of microvascular complications by 50 to 75 percent as compared with conventional regimens. Although no large-scale investigations have been completed, smaller studies have reported similar benefits for intensive therapeutic regimens in patients with type 2 (non–insulin-dependent) diabetes.2
Primary care physicians provide medical care for 75 percent of children and 90 to 95 percent of adults with diabetes.3 Regardless of the type of diabetes, improved glycemic control often can be achieved with individualized tools for patient self-management, carefully formulated nutrition plans and the use of alternative insulin regimens.4
Overview of Insulin
Insulin is necessary for the normal metabolism of carbohydrates, protein and fat. Normal insulin secretion has both basal and meal-stimulated components. Basal insulin secretion, which is usually in the range of 0.5 to 1.0 unit per hour, retards hepatic glucose production in the postabsorptive state.5 The fasting blood glucose level is the base on which prandial glycemia is added during the next 24 hours.6 The meal-stimulated phase of insulin secretion (1 unit of insulin per 10 g of carbohydrate) promotes the dispersal of ingested nutrients, primarily glucose, into the periphery.6 Insulin is also released when blood glucose concentrations exceed 100 mg per dL (5.6 mmol per L).5
In persons who do not have diabetes, insulin is very sensitive to the rise in blood glucose concentration that occurs in response to meals. Endogenous insulin secretion generally peaks within one hour after a meal. Once the meal-stimulated glycemia has subsided, insulin and glucose levels return to premeal levels within two hours. This does not occur in patients with diabetes. Therefore, commonly prescribed regimens consisting of combined short-acting (regular) and intermediate-acting insulins are used to mimic endogenous insulin response. However, these regimens have been incapable of adequately simulating the basal or meal-stimulated components of normal insulin secretion. The physiologic profile of insulin requires rapid changes in concentration as a result of food ingestion or other factors, such as exercise. Furthermore, insulin is a hormone with a half-life of only five to seven minutes.7
Regular human insulin (e.g., Humulin R, Novolin R, Velosulin BR) seldom achieves glycemic control because it contains hexamers of insulin crystallized around a zinc molecule. Although this hexameric insulin is injected subcutaneously, it cannot be absorbed into the bloodstream in this form. Instead, it must first dissociate into dimers and monomers. Dissociation occurs by dilution as the insulin diffuses from the injection site. Diffusion is slow, requiring 50 to 90 minutes, and therefore limits insulin absorption.8 Factors that affect the action of insulin are listed in Table 1.7
| Insulin source* and type |
| Insulin antibodies |
| Insulin dose |
| Injection site |
| Injection technique |
| Exercise |
| Temperature |
The administration of regular human insulin with each meal, a component of intensive diabetes management, is intended to maintain postprandial blood glucose levels as close to normal as possible.4,9 The limitations of regular human insulin therapy are listed in Table 2.5 Many diabetic patients do not consider the importance of timing in administering their insulin injections; instead, they elect to inject insulin at more convenient but inappropriate times. Inappropriate timing of insulin administration results in a mismatching of postprandial carbohydrate absorption and postinjection insulin peak. Regular human insulin is still present in the blood when peripheral glucose disposal occurs. This mismatch predisposes patients to development of acute complications of diabetes such as hypoglycemia.10 Suboptimal glucose control also places patients at risk for long-term microvascular complications, nephropathy, neuropathy and retinopathy.1
Insulin Lispro
Short-acting insulin analogs are designed to overcome the limitations of conventional regular human insulin. Insulin lispro (Humalog), formerly called LYSPRO from the chemical nomenclature [LYS(B28), PRO(B29)], is the first commercially available insulin analog. Compared with regular human insulin, this insulin analog offers the advantages of faster subcutaneous absorption, an earlier and greater insulin peak, and a shorter duration of action.11,12
The benefits achieved by insulin lispro are related to a sequence switch of two beta-chain amino acids. Human insulin, a protein hormone composed of two polypeptide chains, has a linked A chain and B chain. In insulin lispro, reversal of the proline at B-28 and the lysine at B-29 results in more rapid dissolution of this insulin to a dimer and then to a monomer that is absorbed more rapidly after subcutaneous injection6 (Figure 1).13
Pharmacology
The pharmacology of lispro insulin is similar to that of all insulins. Insulin lispro is equipotent to regular human insulin on a molar basis. One unit of this insulin has the same glucose-lowering effect as one unit of regular human insulin.14
Pharmacokinetics and Pharmacodynamics
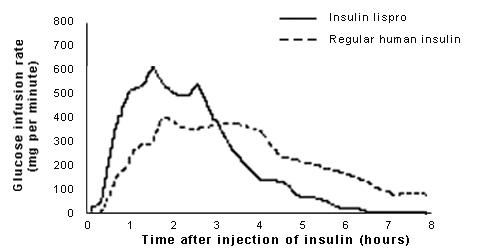
Insulin lispro and regular human insulin have different pharmacokinetics (Table 3).14 Because insulin lispro begins to exert its effects within 15 minutes of administration, patients must eat within this time period. Compared with insulin lispro, regular human insulin has a slower onset of action. Thus, it should be injected 30 to 45 minutes before meals (personal communication from Eli Lilly and Company, based on data on file, September 1997). This time frame allows regular human insulin to reach peak activity at the time of the peak absorption of nutrients from a meal.15 Surveys indicate, however, that patients find it difficult to coordinate the insulin injection time of 30 to 60 minutes before a meal with the actual time that the meal is consumed.16
| Type of insulin | Onset | Peak effect | Duration |
|---|---|---|---|
| Rapid acting: insulin lispro (Humalog) | 0 to 15 minutes | 30 to 90 minutes | Less than 5 hours |
| Short acting: regular human insulin (Humulin R, Novolin R) | 30 to 45 minutes | 2 to 4 hours | 6 to 8 hours |
Greater reductions in postprandial blood glucose excursions have been achieved with insulin lispro administered immediately before meals than with regular insulin given 30 minutes before meals.17–19 When insulin lispro is used, postprandial self blood glucose monitoring should be added to the monitoring schedule.
Peak serum concentrations of insulin lispro occur 30 to 90 minutes after subcutaneous administration. With subcutaneously administered regular human insulin, peak serum concentrations occur within two to four hours. Therefore, regular human insulin therapy may lead to hypoglycemia between meals (i.e., after food has been absorbed but while insulin is still active).
The duration of activity for insulin lispro is less than five hours, compared with six to 10 hours for regular human insulin (personal communication from Eli Lilly and Company, based on data on file, September 1997). With injection in the abdomen, the peak concentration of insulin lispro is slightly higher and the duration of action slightly shorter than when the analog is administered in the arm or thigh. However, insulin lispro is consistently absorbed faster than regular human insulin, regardless of the site of administration.13 As with any insulin preparation, differences in absorption may occur between patients or even in the same patient.
Indications, Dosing and Administration
Insulin lispro is available only by prescription and is indicated for the management of hyperglycemia in patients with diabetes mellitus. Guidelines for glycemic control are listed in Table 4.20,21 Because of its more rapid onset and shorter duration of action, insulin lispro should always be part of a regimen that includes a longer-acting human insulin,5 except when continuous subcutaneous insulin infusion therapy is used.22
Based on product information from Eli Lilly and Company, the dosage of insulin lispro should be individualized, with therapy initiated as outlined in Table 5. Patients who use insulin lispro should monitor their blood glucose levels frequently, especially their postprandial levels. The U.S. Food and Drug Administration has not approved insulin lispro for continuous subcutaneous infusion therapy, although this method has been used in clinical studies. Insulin lispro also is not approved for intravenous or intramuscular administration.
Insulin lispro is physically compatible with Eli Lilly's intermediate-acting human insulins (Humulin N, Humulin L) and longer-acting human insulin (Humulin U). Insulin lispro may be mixed in the same syringe with these insulins, provided that the injection is administered immediately.14 However, the insulin lispro should be drawn into the syringe first so that the vial of short-acting insulin is not contaminated with a longer-acting insulin.21 Predrawn syringes of mixed insulin should not be stored. Not enough information is available to determine whether insulin lispro can be mixed with other insulin types in pre-drawn syringes. Animal insulins or human insulins produced by companies other than Eli Lilly should not be mixed with insulin lispro, because compatibilities have not yet been confirmed.5,14
Insulin lispro is packaged as 100 units per mL, in 10-mL vials, at an average wholesale price of $24.98 per vial, or as five 1.5-mL cartridges at an average wholesale price of $29.99 for five cartridges.16 In contrast, regular human insulin costs $19.84 for 100 units per mL, in 10-mL vials, or $24.11 for five 1.5-mL cartridges.23 Insulin lispro should be kept refrigerated but not frozen. However, it can be left unrefrigerated for up to 28 days, at which time it must be discarded.14
Insufficient information exists concerning the effect of impaired renal or hepatic function on insulin lispro levels. Dose adjustments may be necessary because of the possibility of higher insulin concentrations in patients with renal or hepatic disease.
Adverse reactions
Hypoglycemia can occur if patients do not eat within 15 minutes after receiving insulin lispro. Furthermore, patients may experience postprandial hypoglycemia if the carbohydrate content of a meal is too low. Thus, the dosage of insulin lispro may need to be adjusted for meal composition and size. Late postprandial hyperglycemia can occur if the insulin lispro dosage is decreased and the patient subsequently consumes a low-carbohydrate meal.24
The overall rate of hypoglycemia has not differed for diabetic patients receiving insulin lispro or regular human insulin. However, patients with type 1 diabetes who are treated with insulin lispro have been found to have fewer hypoglycemic episodes between midnight and 6 a.m. than patients treated with regular human insulin.25 The lower rate of hypoglycemia with insulin lispro may be related to higher nocturnal blood glucose levels (due to the insulin's shorter duration of action), as reflected by an increase in morning blood glucose levels.
The DCCT26 established that the incidence of severe treatment-induced hypoglycemia increases significantly with intensive therapy. Severe hypoglycemia is defined as any episode of hypoglycemia that impairs the patient's neurologic function so that the assistance of another person is required.27 Manifestations of severe hypoglycemia can include disoriented behavior, loss of consciousness, inability to be aroused from sleep and/or the occurrence of seizures. Some patients with type 1 diabetes fear severe hypoglycemia as much as the long-term complications of the disease.28 In fact, this fear of hypoglycemia can be a major barrier to achieving glycemic control.26
The magnitude of exercise-induced hypoglycemia with insulin lispro depends on the interval between insulin administration and exercise. Compared with regular human insulin, insulin lispro is more likely to prevent exercise-induced hypoglycemia in patients with type 1 diabetes who choose to exercise two to three hours after a meal.29 If exercise is to be performed soon after food ingestion and insulin administration, the dose of insulin lispro should be decreased. Practical considerations for patients experiencing hypoglycemic reactions are listed in Table 6.
| Consideration | Comments |
|---|---|
| Is the correct amount of insulin being measured and given? | Eyesight deteriorates as a consequence of diabetic and nondiabetic eye disease. |
| Consider having the patient use a pen device* or a magnifying glass that fits around the insulin vial while he or she is drawing up the insulin dose, or have a caregiver or some other person provide assistance in drawing up the dose. | |
| Is the patient rotating injection sites? | Examine the injection sites; if necessary, remind the patient to rotate these sites. |
| Patients with longstanding diabetes often use single injection sites because they seem relatively pain-free; insulin absorption from these sites is notoriously variable. | |
| Is the patient performing regular blood glucose monitoring? | Instruct the patient to monitor blood glucose levels frequently at the time of a change in insulin therapy. |
| Check the patient's monitoring technique for common mistakes, including not having sufficient blood on the stick, incorrect wiping of the sample and incorrect timing before the stick is read. | |
| The newer self-monitoring devices have eliminated most errors of timing and wiping, and some even tell the patient that insufficient blood is on the stick. |
Rates of insulin allergy, lipodystrophy, hypoglycemia and abnormal laboratory test results have not differed in patients using insulin lispro or regular human insulin.30
Drug Interactions
No studies have specifically evaluated drug interactions in diabetic patients who are receiving lispro insulin. Close monitoring of blood glucose levels is important when a drug regimen is changed in any patient with diabetes.
Therapeutic Role
Insulin lispro has been found to be a safe and effective treatment for diabetes mellitus. Improvement in glycemic control is demonstrated by a decreased postprandial blood glucose concentration, although the clinical significance of this improvement is as yet unknown.
Multinational clinical trials have shown no statistically significant difference between hemoglobin A1c levels in patients treated with insulin lispro and patients treated with regular human insulin.5,17 However, the use of insulin lispro in external insulin infusion pumps has been shown to produce a small, yet clinically significant (0.34 percent) reduction in hemoglobin A1c levels compared with the reduction achieved using regular human insulin. Based on risk analysis of the DCCT data, this improvement in hemoglobin A1c represents an approximately 20 percent reduction in the risk of retinopathy in patients with diabetes.22
Near-normal glycemic control is necessary to prevent or delay the onset of complications in patients with type 1 or type 2 diabetes. Patients with type 2 diabetes who have not responded to oral glucose-lowering agents often require insulin therapy to achieve the glycemic goals set forth by the American Diabetes Association. One study31 in both type 1 and type 2 diabetics concluded that insulin lispro improves postprandial glycemic control without increasing the risk of hypoglycemia.32 Short- or long-term insulin therapy has been shown to be useful in type 2 diabetics in whom the rapid component of endogenous insulin secretion is missing.32 In these patients, insulin lispro is a physiologic therapy. Special dosing considerations for insulin lispro are listed in Table 7.33
| Potential problem | Comments and possible solutions |
|---|---|
| Patient eats dinner late | Because of insulin lispro's shorter duration of action, hyperglycemia may occur because the time from lunch to dinner may be too long. |
| Consider adding a small dose of intermediate-acting (NPH) insulin at lunch to meet basal insulin requirements between meals. | |
| Patient has snacks containing more than 5 g of carbohydrate | Consider adding an additional dose of insulin lispro; if the patient also eats dinner late in the evening, this additional dose of insulin lispro can replace lunchtime basal NPH insulin supplementation. |
| Patient is a slow eater or a grazer (i.e., eats small amounts of carbohydrates throughout the day rather than at three meals) | Because of the rapid onset of insulin lispro, this type of patient may not respond as well to insulin lispro as to regular human insulin. |
| Patient has unpredictable eating habits | Insulin lispro offers the patient flexibility, in that the administration of this insulin can be timed with meals. |
| Patient has type 2 diabetes and is receiving two injections of NPH/regular insulin each day | This patient could benefit from the substitution of insulin lispro for regular human insulin to decrease postprandial blood glucose excursions. |
| Patient exercises | The patient who uses insulin lispro can expect fewer episodes of hypoglycemia if the exercise is undertaken 2.0 to 2.5 hours after the injection of insulin lispro. |
Primary care physicians should consider including mealtime insulin lispro in insulin regimens. The disadvantages of insulin lispro therapy are the increased risk of hypoglycemia if meal ingestion or absorption (gastroparesis) is delayed and the increased overall cost of therapy. In addition, insulin lispro is available by prescription only. Nonetheless, a short-acting insulin analog such as insulin lispro should provide increased convenience and flexibility to patients who are currently receiving regular human insulin. Furthermore, the characteristics of insulin lispro may help patients achieve improved long-term glycemic control and may reduce the incidence of hypoglycemic episodes. Insulin analogs may be an important tool for helping patients with diabetes mellitus achieve their target glucose goals.