
This is a corrected version of the article that appeared in print.
Am Fam Physician. 1998;57(2):315-322
See related patient information handout on taking medicine for HIV disease, written by the authors of this article.
In 1996 a panel of experts convened by the International AIDS Society–USA issued new guidelines for treating human immunodeficiency virus (HIV) infection, which have recently been updated. Quantitative plasma HIV-1 RNA concentration (viral load) and CD4+ lymphocyte levels are used to monitor disease progression, determine the need to initiate antiretroviral treatment, monitor effectiveness of treatment and evaluate the need to change medications. Multi-drug therapy with nucleoside analogs, nonnucleoside reverse transcriptase inhibitors and protease inhibitors can result in measurable improvement in clinical outcome in HIV-1 infected patients.
Advances in the treatment of human immunodeficiency virus (HIV) disease have made the antiretroviral therapy guidelines issued in 1993 inapplicable to current clinical decision making. A panel of experts for the International AIDS Society–USA developed new recommendations that were presented at the XI International Conference on AIDS in Vancouver.1 The panel addressed four issues: when to initiate therapy, which types of drugs to use, when to change therapy and which types of drugs to use when a change in therapy is indicated. Recently updated, these recommendations represent current thinking on the management of HIV disease.2
Viral Load Testing
In the past, CD4+ lymphocyte counts provided the basis for the initiation of antiretroviral treatment and were used to help define acquired immunodeficiency syndrome (AIDS). Although produced in high numbers in response to HIV infection, CD4+ lymphocytes are the principal targets for replication of the HIV virus. The virus enters the cell through various receptors, replicates inside the cell and kills the cell as the viral progeny burst the cell walls to seek other CD4+ lymphocytes.3 We now know that the CD4+ lymphocyte is only an intermediate variable in a chain of variables leading from viral replication to the development of AIDS; measurement of the CD4+ lymphocyte count is only an indirect marker of antiretroviral therapy, a measurement of the relative production and destruction of CD4+ cell population.
Viral load, a measurement of the level of circulating (plasma) HIV RNA, reported in copies per mL, is a direct reflection of ongoing viral replication. As such, it is superior to the CD4+ lymphocyte count for predicting the rate of progression to development of AIDS and for monitoring the effects of antiretroviral treatment. CD4+ counts assist decision making about prophylactic therapies against opportunistic infections, since they reflect the body's ability to fight off infection in general. Figure 1 illustrates the time course of HIV disease and the relative levels of circulating viral RNA and CD4+ lymphocytes. In general, as the amount of virus increases, the CD4+ count decreases, although this inverse relationship is neither constant nor predictable. The level of HIV RNA can rise or fall much more quickly than the CD4+ count.
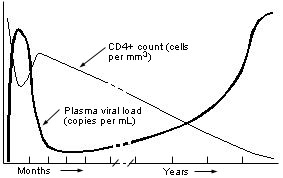
During the initial infection, viral replication increases dramatically, then declines to a steady state during the chronic, asymptomatic phase of HIV illness. The latter phase can last from months to years before the infection progresses to the symptomatic phase. The viral load in the chronic, asymptomatic period is fairly stable in each person but varies greatly from one person to another. The HIV RNA level has been shown to have prognostic value in predicting disease progression and time to death: the higher the level, the faster the rate of progression.4,5
Currently, three assays are available for determining viral loads in HIV infection: (1) Branched DNA (bDNA; Quantiplex—manufactured by Chiron Therapeutics, Emeryville, Calif.). This assay uses a signal amplification technique. After high-speed centrifugation, the virus releases its RNA. Specific probes bind the RNA and adhere it to a special plate. Following a series of hybridization steps, multiple bDNA and alkaline phosphatase–labeled molecules bind to the immobilized HIV RNA. A single HIV RNA may bind up to 1,755 alkaline phosphatase molecules, greatly enhancing the detection signal. A chemiluminescent substrate is added; light emission is proportional to the amount of RNA present. (2) Reverse transcriptase polymerase chain reaction (RT-PCR; Amplicor HIV-1 Monitor—manufactured by Roche Diagnostic Systems, Inc., Somerville, N.J.). Viral RNA is reverse transcribed into a double-stranded DNA molecule. The two strands are then separated, nucleotides are added and more double-stranded DNA molecules are created. After a known number of copies are produced, a known number of internal control molecules are added, and the ratio of the detection signal to the internal standard detection signal is calculated. (3) Nucleic acid sequence–based amplification (NASBA—manufactured by Organon Teknika but not yet commercially available). This assay is also a nucleic acid amplification method but rather than amplifying DNA, as does the Amplicor HIV Monitor Test, the NASBA assay amplifies RNA. The advantage of this assay is that it can measure viral loads in any tissue, not just plasma.
The first two assays are compared in Table 1. The cost per viral load assay is $160 to $240. In general, the assays give similar results but, despite reportedly good between-test agreement, it is recommended that a new baseline be obtained when switching assays. To be considered significant, a change in viral RNA must be at least twofold (0.3 log10), since this is the normal variation of levels in the clinically stable patient. This level of change takes into account intralaboratory and patient variability.
When Should Viral Load Testing Be Performed?
Viral load testing plays a role in four strategic management issues: assessing the risk of disease progression, determining when to initiate antiretroviral therapy, evaluating the effectiveness of an antiretroviral regimen and determining when a drug regimen is failing. As with other clinical decisions, viral load testing should not be performed if the results will not affect management. Table 2 summarizes the critical decision points in the management of HIV disease and the recommendations for using viral load testing at these points.
| Decision point | Recommendations | ||
|---|---|---|---|
| Assessing risk of disease progression | |||
| Establishing a baseline | Obtain two measurements of viral load and CD4+ lymphocyte count 2 to 4 weeks apart | ||
| Monitoring the asymptomatic patient | Obtain a viral load assay and CD4+ lymphocyte count every 3 to 6 months, with shorter intervals as critical decision points approach | ||
| Determining when to initiate antiretroviral therapy | The patient is symptomatic,* | ||
| or | |||
| The patient is asymptomatic | |||
| and | |||
| CD4+ count is <500 per mm3 (500 × 106 per L) | |||
| or | |||
| CD4+ count is >500 per mm3 (500 × 106 per L) | |||
| and | |||
| Viral load is >30,000 copies per mL† | |||
| or | |||
| CD4+ count is dropping rapidly (loss of >300 per mm3 [300 × 106 per L] over 12 to 18 months) | |||
| Evaluating the effectiveness of an antiretroviral regimen | Monitor viral load 3 to 4 weeks after initiating or changing therapy | ||
| Follow viral load every 3 to 6 months along with CD4+ lymphocyte count (viral load should decline by at least 68 % [0.5 log10]) | |||
| Determining when a drug regimen is failing | When the viral load returns to within 32 to 68 % of the pretreatment baseline and the patient has not had an immunization in the past month and is not currently ill with an active intercurrent infection | ||
| Confirm with two measurements 2 to 4 weeks apart | |||
Assessing the Risk of Disease Progression. In establishing a baseline viral load in the clinically stable patient, it is preferable to obtain two measurements two to four weeks apart, along with a baseline CD4+ count. In the chronic, asymptomatic stage, viral load is a strong predictor of rapid progression to AIDS, independent of the CD4+ count. Only 59 percent of patients whose viral load after seroconversion was less than 4,500 copies per mL progressed to AIDS over the following five years.4 A level above 100,000 copies per mL was the most powerful predictor of AIDS, with an odds ratio of 10.8. Table 36 illustrates the risk of developing AIDS over 10 years. Viral load testing should not be used for diagnostic purposes. If the ELISA and Western Blot tests are equivocal and it is thought that the patient may be in the “window” period before significant antibodies are detected, a test for the p24 antigen should be used.
Determining When to Initiate Antiretroviral Therapy. If the patient is symptomatic or the CD4+ count is less than 500 per mm3 (500 × 106 per L), therapy is recommended regardless of the viral load. Until recently, it was also recommended that therapy be started in asymptomatic patients with a CD4+ count greater than 500 per mm3 (500 × 106 per L) and a viral load greater than 30,000 copies per mL. Now, however, earlier initiation is recommended, to include patients whose viral load is greater than 5,000 to 10,000 copies per mL.2 Treatment at this early stage has not been supported directly by clinical data, and physicians must consider the potential problems of drug toxicity, drug intolerance, expense and the potential emergence of drug-resistant virus.
Evaluating the Effectiveness of an Antiretroviral Regimen. After initiating antiretroviral therapy, the effectiveness of treatment can be assessed by monitoring viral load and CD4+ counts in three to four weeks and every three to six months thereafter. In general, the viral titer should decline to at least 32 percent (≈0.5 log10) of the pretreatment value, with the goal of an undetectable level.
Determining When a Drug Regimen Is Failing. Increased viral replication suggests drug-resistance and is manifested by a return of the viral titer and CD4+ count toward the pre-treatment level, as well as a progression of symptoms. When the viral load increases to 2,000 to 5,000 copies per mL from undetectable levels (or to 5,000 to 10,000 copies per mL if treatment levels were previously below this range),2 a change in therapy is recommended. Physicians must use caution, however, when interpreting a rising viral titer if the patient has an active intercurrent illness or has had immunizations within the previous month. For unknown reasons, viral loads can increase dramatically at such times.
Initial Medications
Several nucleoside analogs, nonnucleoside reverse transcriptase inhibitors and protease inhibitors are available7,8 (Tables 4 through 6). Nonnucleoside reverse transcriptase inhibitors bind to reverse transcriptase, inhibiting conversion of viral single-stranded RNA to double-stranded DNA, and nucleoside analogs incorporate themselves into the double-stranded DNA. Protease inhibitors act later in the life cycle to prevent viral proteins from being packaged into infectious virions (Figure 2). The International AIDS Society–USA panel recommends that therapy be initiated with at least two nucleoside analogs. Monotherapy is not recommended. A protease inhibitor should be included in the treatment regimen of patients at higher risk for progression to AIDS (i.e., those with a rapidly falling CD4+ count, a viral load greater than 100,000 copies per mL or symptoms, or according to the clinician's judgment).
| Drugs and availability | Daily dosage | Major toxicities and concerns | Cost* | Monitoring |
|---|---|---|---|---|
| Zidovudine (AZT, ZDV; Retrovir), 100 mg, 300 mg capsules; 50 mg per 5 mL syrup; 10 mg per mL IV infusion | Adults: 200 mg every 8 hours or 300 mg every 12 hours Children 3 months to 12 years: 180 mg per m2 every 6 hours; maximum: 200 mg every 6 hours | Bone marrow suppression (severe anemia, granulocytopenia, thrombocytopenia), myopathy, myositis | 100 mg, #180, $2 87 300 mg, #60, $287 Syrup: 8 oz, $38 | Periodic CBC |
| Didanosine (ddI; Videx), 25 mg, 50 mg, 100 mg, 150 mg chewable tablets; 100 mg, 167 mg, 250 mg powder packets; powder for oral solution: 2 g, 4 g | Tablets, powder packets: | Pancreatitis, peripheral neuropathy, liver failure, retinal depigmentation and change in vision (in children) Must be taken on empty stomach, 2 hours after or 1 hour before food. | 100 mg, #120, $186 250 mg packets, #60, $233 2 g powder, $31 4 g powder, $62 | Children: dilated retinal examination every 6 months |
| ≥60 kg: 200 mg every 12 hours | ||||
| <60 kg: 125 mg every 12 hours | ||||
| Oral solution: | ||||
| ≥60 kg: 250 mg every 12 hours† | ||||
| <60 kg: 167 mg every 12 hours† | ||||
| Zalcitabine (DDC; Hivid), 0.750 mg, 0.375 mg tablets | ≥30 kg: 0.75 mg every 8 hours <30 kg: 0.375 mg every 8 hours | Peripheral neuropathy (22 to 35%), pancreatitis, esophageal ulcers, stomatitis, cardiomyopathy, hepatic failure | 0.75 mg, #90, $207 0.375 mg, #90, $165 | Periodic CBC, chemistry profile, amylase determination |
| Lamivudine (3TC; Epivir), 150 mg tablets; 10 mg per mL oral solution | >50 kg: 150 mg every 12 hours‡ | Headache, insomnia, fatigue, peripheral neuropathy, myalgia, pancreatitis | 150 mg, #60, $230 Oral solution: 8 oz, $61 | — |
| <50 kg: 2 mg per kg every 12 hours‡ | ||||
| 3 months to 12 years: 4 mg per kg twice daily | ||||
| Stavudine (D4T; Zerit), 15 mg, 20 mg, 30 mg, 40 mg capsules; 1 mg per mL oral solution | ≥60 kg: 40 mg every 12 hours | Peripheral neuropathy (15 to 21%), hepatotoxicity, anemia, pancreatitis | 40 mg, #60, $244 30 mg, #60, $235 | Periodic liver function tests∥ |
| 30 to 59 kg: 30 mg every 12 hours | ||||
| <30 kg: 1 mg per kg every 12 hours |
| Drugs | Daily dosage | Major toxicities and concerns | Cost* | Monitoring |
|---|---|---|---|---|
| Nevirapine (Viramune), 200 mg tablets | 200 mg every day for 14 days; then increase to 200 mg twice daily | Rash, nausea, vomiting, diarrhea, fatigue, fever, elevated liver enzymes, Stevens-Johnson syndrome | 200 mg, #60, $248 | Liver function tests |
| Delavirdine (Rescriptor), 100 mg tablets | 400 mg three times daily | Rash, nausea, headache, elevated liver enzymes (worse when taken in combination with saquinavir [Invirase]); 4 tablets can be stirred into a slurry in ≥8 oz water; drink immediately | 100 mg, #360, $222 | — |
| Drugs | Daily dosage | Major toxicities and concerns | Cost* | Monitoring |
|---|---|---|---|---|
| Saquinavir (SQV; Invirase), 200 mg capsules | 600 mg every 8 hours | Diarrhea, nausea, abdominal discomfort, elevated transaminase levels | 200 mg, #270, $572 | Periodic chemistry profile |
| Take with high-fat meal or within 2 hours of a full meal | ||||
| Ritonavir (Norvir), 100 mg capsules; 80 mg per mL oral solution | 600 mg every 12 hours | Diarrhea, nausea, taste perversion, vomiting, anemia, increased hepatic enzymes, increased triglycerides | 100 mg, #360, $668 8 oz, $312 | Periodic liver function tests |
| Requires refrigeration; take with meals; chocolate milk improves the taste | ||||
| Indinavir (Crixivan), 200 mg, 400 mg capsules | 800 mg every 8 hours | Hyperbilirubinemia, nephrolithiasis; take 1 hour before or 2 hours after food; may take with skim milk or a low-fat meal; drink >1.5 L of liquid daily | 400 mg, #180, $450 | — |
| Nelfinavir (Viracept), 250 mg tablets, 50 mg per g powder | 750 mg three times daily with meals | Diarrhea (16 to 32%), nausea, flatulence, rash | 250 mg, #270, $580 | — |
| 20 to 30 mg per kg per dose, three times daily with meals† |
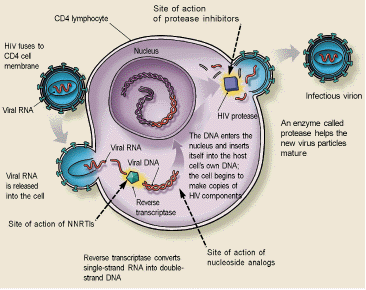
Dosages and toxic effects of the various antiretroviral drugs are listed in Tables 4 through 6. These medications are expensive and should be taken as regularly as possible (e.g., every eight hours rather than three times a day—see patient information handout). Based on average wholesale prices, the annual cost of treatment with nucleoside analogs can range from $2,332 to $3,442; the range for treatment with nonnucleoside reverse transcriptase inhibitors is about $5,400 to $8,014, and for protease inhibitors, $2,663 to $2,967. Physicians are advised to become familiar with the interactions and adverse reactions of these agents before prescribing them.
Changing Medications
The main reasons for considering a change in initial therapy are treatment failure and drug-related problems (toxicity, intolerance or noncompliance). Treatment failure is evidenced by either increased viral replication, evidenced by a rise to greater than 2,000 to 5,000 copies per mL if previous levels were undetectable or an increase to greater than 5,000 to 10,000 copies per mL if previous levels were below this level.2 Measuring viral load three to four weeks after initiating or changing therapy and every three to six months thereafter is recommended. Viral suppression may take many months in patients with high viral loads. Dose-limiting toxicity occurs more frequently as the disease progresses, both as a direct effect of the disease and as a consequence of interactions with other medications the patient may be taking.
When changing therapy, the new regimen should include a combination of at least two new drugs (Table 7). If the patient started with two nucleoside analogs, the next recommended regimen would be to change both or to change one and add a protease inhibitor. If the initial regimen included a protease inhibitor, subsequent regimens would still adhere to the rule of two new drugs.
| Initial treatment options* | Then switch to* | Viral load >100,000 copies/mL |
|---|---|---|
| Zidovudine (Retrovir) + lamivudine (Epivir) | Didanosine + stavudine | Include a protease inhibitor or an NNRTI |
| Stavudine (Zerit) + didanosine (Videx) or zalcitabine (Hivid) | Zidovudine + lamivudine | Include a protease inhibitor or an NNRTI |
| Stavudine + lamivudine | Zidovudine + didanosine or an NNRTI | Include a protease inhibitor |
| Zidovudine + didanosine | Stavudine + lamivudine | Include a protease inhibitor or an NNRTI |
Development of Drug Resistance
Antiretroviral resistance is a common problem. Spontaneous mutation occurs once in every 104 replications. It is estimated that 1010 replicative cycles occur daily.9 The only good way to keep resistance from developing is to keep the virus from replicating. Multi-drug therapy attacks the virus from different directions and is better than monotherapy at keeping the viral load down. If a patient decides to stop taking the medication, it may be best to stop taking all antiretroviral medications rather than to use monotherapy. Professional judgment and an understanding of the patient's priorities are necessary in this difficult situation.
Upcoming Recommendations
New recommendations that were anticipated in late 1997 will be published in the Morbidity and Mortality Weekly Report. These recommendations will possibly urge clinicians to initiate therapy with three antiviral agents (two nucleoside analogs plus one protease inhibitor or two nucleoside analogs plus one nonnucleoside reverse transcriptase inhibitor) when the CD4+ count is less than 350 per mm3 (350 × 106 per L) regardless of the viral load, or when the CD4+ count is between 351 and 500 per mm3 (351 and 500 × 106 per L) and the viral load is greater than 10,000 copies per mL.10
Final Comment
Recent laboratory progress in determining HIV-1 RNA viral concentration has allowed a more aggressive approach in the treatment of HIV infection. Further drug development promises breakthroughs in aborting viral replication. The new recommendations and drug protocols are not all based on clinical trials, but many represent expert opinion.