
Am Fam Physician. 1998;57(3):504-510
Amnioinfusion is being used to treat intrapartum problems known to be associated with fetal compromise, including prophylactic treatment of oligohydramnios during labor and after premature rupture of the membranes, treatment of severe variable decelerations during labor and reducing the risk of meconium aspiration during labor in patients with thick meconium fluid. The procedure is considered effective and easy to perform, with the benefits outweighing the risks.
Amnioinfusion is a relatively new technique with several applications. In various obstetric situations, when lack of amniotic fluid is perceived to be the problem, the infusion of fluid into the amniotic cavity is a simple and logical treatment approach. The two most common applications are treatment of severe variable decelerations in fetal heart rate and dilution of thick meconium fluid during labor. Artificially increasing amniotic fluid volume may better protect the umbilical cord from compression, and thus may reduce the number and severity of variable decelerations. Diluting thick meconium fluid may reduce the risk of meconium aspiration syndrome. Amnioinfusion may allow spontaneous vaginal delivery and avoid the necessity of operative intervention. This inexpensive technique appears to pose little risk and warrants consideration in properly selected patients.
Amnioinfusion was first described in 1976.1 Using a rhesus monkey model, the authors reported that variable decelerations occurred when amniotic fluid was removed from the uterine cavity and resolved when it was replaced. Although this experiment established that variable decelerations related to oligohydramnios and cord compression could be corrected by amnioinfusion, the technique did not achieve clinical application until 1983, when a novel approach to the relief of variable or prolonged decelerations was described.2
Indications
Severe variable decelerations and thick meconium fluid are the two main indications for transcervical amnioinfusion.
Severe Variable Decelerations
Variable deceleration is the most common periodic pattern noted during labor. It represents a normal response to decreased umbilical blood flow. The deceleration is a vagal reflex–mediated change in fetal heart rate, generally caused by umbilical cord compression that may occur as a result of the cord being around the neck or under the arm of the fetus, or between some part of the fetus and the uterine wall. Variable deceleration is defined as a fetal heart rate pattern of decelerations that are variable in shape and relationship to contractions (Figure 1). Decelerations generally occur with a contraction and have a rapid, sharp descent and recovery. Variable decelerations may be classified as mild, moderate or severe (Table 1). They have both benign and ominous indicators (Table 2).
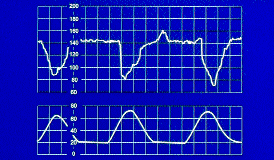
Variable decelerations that are mild and not repetitive are usually associated with a good fetal outcome. However, this otherwise normal response to decreased umbilical blood flow may worsen as labor progresses. With moderate and severe variable decelerations, or decelerations with ominous indicators, a significant reduction in umbilical blood flow may occur. If variable decelerations become severe and persist, the fetal condition can deteriorate. If severe variable decelerations or ominous indicators cannot be corrected, emergency operative delivery may be required.
Variable decelerations are generally associated with a favorable outcome. Those occurring before fetal descent at 8 to 9 cm of dilatation occur most frequently in patients with oligohydramnios. Only when they become persistent, progressively deeper and longer lasting, and are associated with ominous indicators, are they considered nonreassuring.
The response of the baseline fetal heart rate to the variable decelerations and the presence or absence of accelerations is important to the physician in formulating a management plan for the patient with significant variable decelerations. If the baseline variability and fetal heart rate remain stable, or are only minimally affected, conservative maneuvers designed to alter the geometric relationship between the fetus, the cord and the uterine wall are implemented (e.g., changing the mother's position). The response of variable decelerations to corrective measures is fairly unpredictable. If these management measures do not correct pattern severity, amnioinfusion may be useful in reducing the number of operative interventions.
In 1985, a prospective, randomized study3 of 96 cases of repetitive variable fetal heart rate decelerations not relieved by changes in maternal position or oxygen administration was published. Patients were randomly assigned to a saline amnioinfusion group or a control group. Patients within each group were further stratified by parity. Outcome measurements included complete relief of variable decelerations, cesarean section rate for fetal distress and infant Apgar scores. Statistically significant differences were noted in the relief of variable decelerations in all patients in the infusion group and in the cesarean section rate in the nulliparous patients but not in the multiparous patients who received amnioinfusion. An explanation for this disparity is that multiparous patients in an active advanced first stage of labor can be expected to deliver shortly and brief fetal distress is more easily tolerated, whereas the opposite is true in nulliparous patients. Apgar scores were similar in both the study and control groups.
No large prospective controlled trials have been reported subsequently. Current supportive evidence for this procedure derives from meta-analysis of the nine randomized trials reported.4 Amnioinfusion was effective in preventing or relieving fetal heart rate decelerations. The main clinical benefit was a large reduction in the number of cesarean sections performed for the indication of non-reassuring fetal heart rate tracing. It is likely that the latter diagnoses were made on the basis of fetal heart rate patterns, as there was no discussion of the use of fetal scalp blood sampling. These trials should therefore be interpreted in the context of a clinical situation in which the diagnosis of fetal distress is usually made on the basis of fetal heart rate patterns and, under these circumstances, amnioinfusion has the important benefit of reducing the rate of cesarean section.4 A trend toward reduction in operative vaginal deliveries has been seen, as well as a reduction in low Apgar scores at one minute and low cord arterial pH values, in addition to a reduction in postpartum endometritis, which may be secondary to the reduction in cesarean sections.
The groups studied were too small to support any conclusions concerning less common outcomes related to amnioinfusion, such as umbilical cord prolapse, neonatal sepsis and perinatal deaths. The latter issues will only be resolved by the results of large randomized trials.
Thick Meconium Fluid
The passage of thick meconium in utero puts the neonate at risk for meconium aspiration syndrome. Meconium aspiration syndrome develops in 1.8 to 18 percent of infants delivered from meconium-stained amniotic fluid and is associated with increased perinatal morbidity and mortality.5 Aspiration of meconium is considered an intrauterine event, although it can also occur during delivery with the initial breaths of the baby.6–8 In fetuses with oligohydramnios and cord compression, aspiration of meconium occurs as a result of hypoxia and hypercapnia, which act synergistically to stimulate fetal gasping.5
One study8 evaluated patients with meconium passage during early and late labor and graded the presence of meconium as light or heavy. Heavy meconium passage early in labor was associated with increased fetal morbidity, including abnormal heart rate patterns (late decelerations, severe variable decelerations and decreased variability), a prolonged second stage of labor and an increased incidence of operative intervention, including forceps delivery and cesarean section, as well as a significantly higher incidence of neonatal morbidity and mortality, particularly in association with meconium aspiration. Meconium aspiration syndrome accounts for 2 percent of all perinatal deaths.9
The risk of meconium aspiration is high in patients with thick meconium, particularly when it is associated with episodes of fetal hypoxemia. Thin meconium is not associated with an increased perinatal mortality rate or with an increased incidence of meconium aspiration syndrome. Therefore, any mechanism by which thick meconium can be converted to thin meconium in the already potentially compromised fetus is postulated to have a positive affect on neonatal outcome—specifically, a decreased incidence of meconium aspiration syndrome.10,11
The combined obstetric-pediatric approach (perineal suctioning/intubation) to infant tracheal suctioning at delivery was described in a study12 that assumed that most meconium aspiration occurs during delivery. Subsequently, however, it has been well documented that the combined technique of aggressive neonatal suctioning at delivery failed to eliminate all cases of meconium aspiration syndrome. A 2 percent incidence of this syndrome that persisted despite suctioning at delivery is believed to be due to fetal gasping and antenatal aspiration of thick meconium fluid.13
If the presence of meconium below the vocal cords and neonatal acidemia increase the risk for meconium aspiration syndrome, prevention of these complications may reduce the severity of meconium aspiration syndrome. Because the presence of thick meconium indicates a reduction in the volume of amniotic fluid in which the meconium is diluted, it is difficult to differentiate the effects of amnioinfusion that may be the result of dilution of meconium from those that result from replenishment of amniotic fluid volume.12 Amnioinfusion theoretically restores normal amniotic fluid volume and cushions the umbilical cord, which reduces cord compression, allowing for the resumption of normal function as demonstrated by net efflux of aspirated meconium and amniotic fluid in the previously hypoxic or asphyxiated fetus.13
Most studies have reported a decrease in newborn respiratory complications resulting from meconium aspiration in patients who receive amnioinfusion. The results of a meta-analysis14 of six small randomized trials of amnioinfusion for meconium staining demonstrated a reduction in heavy meconium staining, meconium below the vocal cords, meconium aspiration syndrome and the need for neonatal ventilation.
These results are promising yet remain controversial. Two studies question the routine use of amnioinfusion for meconium. In one study,14 it was argued that although each of the studies in the meta-analysis randomized patients to either an amnioinfusion group or a control group (with no amnioinfusion), patients in the control groups did not receive amnioinfusion even when otherwise indicated for relief of variable fetal heart rate decelerations. Thus, control patients may have been predisposed to fetal distress, with subsequent hypoxia and vagal stimulation, fetal gasping and meconium aspiration.
Spong and colleagues15 addressed this issue in a prospective, randomized study in which patients assigned to a control group received “standard care,” which included amnioinfusion for repetitive variable decelerations. There was no difference in outcome between the prophylactic-amnioinfusion group and the standard-care group. They concluded that the benefit of amnioinfusion in patients with meconium-stained amniotic fluid may be the result of alleviation of variable fetal heart rate decelerations, rather than meconium dilution.
The routine use of amnioinfusion for meconium dilution has been questioned.10 In one study, despite a policy of amnioinfusion for patients with thick meconium, not all patients received amnioinfusion (because of lack of time or emergency delivery). In a comparison of the two groups, no difference was found in the incidence of meconium aspiration syndrome.
Although prophylactic amnioinfusion is quickly becoming an accepted standard treatment in patients with thick meconium, controversy persists as to whether further randomized studies that include standard care (i.e., therapeutic amnioinfusion for variable decelerations) in the control group are needed to evaluate the benefit.
Risks
Amnioinfusion has been considered effective, easy to perform and safe. Indications and contraindications for amnioinfusion are listed in Table 3. Few acute events have been attributed to amnioinfusion. Isolated cases of umbilical cord prolapse have been reported, but they were well within the quoted occurrence rate of prolapse in pregnancies with vertex presentation where amnioinfusion was not used.2,16 Other reported infrequent complications of amnioinfusion include one case of uterine scar disruption and one case of iatrogenic polyhydramnios and elevated intra-uterine pressure during amnioinfusion, which led to fetal bradycardia.17 Five cases of amniotic fluid embolism have been reported in the medical literature.18,19 All were associated with other previously reported risk factors for amniotic fluid embolism.
| Indications |
| Repeated severe variable fetal heart rate decelerations not responsive to conventional therapy |
| Thick/particulate meconium staining of the amniotic fluid |
| Contraindications |
| Amnionitis |
| Polyhydramnios |
| Uterine hypertonus |
| Multiple gestation |
| Known fetal anomaly |
| Known uterine anomaly |
| Severe fetal distress |
| Nonvertex presentation |
| Fetal scalp pH <7.20 |
| Placental abruption or placenta previa |
Electrolyte abnormalities after amnioinfusion occurred in animal models; however, in one study,20 no significant changes were found in newborn serum electrolyte levels. One incidence of a temporal relationship between amnioinfusion and the unexpected development of respiratory failure in a healthy parturient has been reported18; however, cause and effect were not established.
These reports have raised questions regarding the safety of amnioinfusion. In a survey of all teaching hospitals in the country to determine how, when and with what result amnioinfusion is performed in the United States,21 it was found that neither the method employed or the number of infusions performed appeared to significantly increase the risk of complication. Although the survey did not address the efficacy of the various protocols employed, it did suggest that all are relatively safe. The fact that the mean number of amnioinfusions performed per year is similar between centers that did and centers that did not report complications suggests that complications are generally infrequent or, perhaps, that the complication incidence decreases as clinician experience increases.
There are failures related to amnioinfusion. The possible causes for failure are inadequate infusions, rapid progression to second stage of labor and cord complications; however, the cause of the greatest majority of failures of the procedure to improve outcomes is unknown.
Although reports of severe or morbid complications associated with amnioinfusion have been rare, these complications are still a cause for concern. The greatest attractions of amnioinfusion have been that it is relatively easy to perform, inexpensive and safe. Even clinicians who think that the benefits of amnioinfusion are subtle have adopted use of the procedure because the benefits seem to clearly outweigh the perceived risks to the mother and fetus.
Transcervical Amnioinfusion Protocol
The procedure is straightforward and uses equipment found in most hospital labor and delivery suites, including the following: a double lumen intrauterine pressure catheter, normal saline solution at room temperature, a fetal monitor and intravenous tubing (Figure 2). Although not required, continuous close monitoring using a fetal scalp electrode is recommended. To date, no benefit has been demonstrated for the use of infusion pumps or solution warmers.22
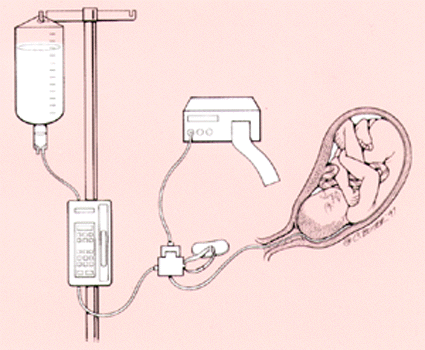
After obtaining informed consent, a vaginal examination is performed to evaluate for cord prolapse, establish dilatation and confirm presentation. The fetal scalp electrode is placed, followed by an intrauterine pressure catheter to document resting tone (< 15 mm Hg). The normal saline is linked to the intravenous tubing. The tubing is primed as it would be for intravenous use. The tubing is then inserted into the infusion port on the three-way stop cock of the intrauterine pressure catheter.
Recommendations for infusion protocols may vary by institution. The more common protocol starts with an initial bolus of 250 mL infused over 20 to 30 minutes. The rate is then adjusted according to the severity of decelerations, but usually at a rate of 10 to 20 mL per minute up to 600 mL, or to resolution of the variable decelerations. An additional 250 mL beyond the volume at which decelerations resolve is administered, then the infusion is terminated, unless the decelerations resume. The infusion is a failure if infusion of 800 to 1,000 mL of saline does not result in termination of decelerations. In patients with thick meconium fluid, an infusion of 250 to 500 mL over 30 minutes, followed by a constant infusion at 60 to 180 mL per hour, is the accepted protocol.
The fetal heart rate and resting tone are assessed continuously during the intervention. If the uterine tone is persistently elevated, discontinue the infusion and allow the uterine pressure to equilibrate over five minutes. Reassess the resting uterine tone. Discontinue the infusion if the new resting tone is 15 mm Hg above the baseline resting tone or 30 mm Hg maximum. Some fluid will leak from the uterine cavity throughout this process in most cases.
Final Comment
Intrapartum amnioinfusion appears to improve outcomes in gravidas with variable decelerations or thick meconium during labor. The procedure has been used since 1983 and is now an accepted therapeutic measure. Many protocols for amnioinfusion are used across the country; they all appear to be safe and associated with few complications. New clinical applications for amnioinfusion continue to be proposed. Future attention should be directed toward ensuring that the evidence of effectiveness of amnioinfusion in specific circumstances is translated into clinical practice and that the remaining unanswered questions are addressed in appropriately sized clinical trials.