
Am Fam Physician. 1998;57(6):1285-1289
See related patient information handout on bacterial vaginosis, written by the author of this article.
Bacterial vaginosis is the most common cause of vaginal discharge. Recent studies have confirmed its association with pelvic inflammatory disease and adverse pregnancy outcomes. Bacterial vaginosis is treated with oral metronidazole (given either as a single dose or a seven-day course) or clindamycin. Treatment with topical clindamycin or metronidazole is also effective in returning the vaginal flora to normal but may be less effective in preventing the increased incidence of adverse pregnancy outcomes.
Bacterial vaginosis, previously known as nonspecific vaginitis or Gardnerella vaginitis, is the most common cause of vaginal discharge. It may be the cause of up to one half of cases of vaginitis1 in all women and the cause of from 10 to 30 percent of cases in pregnant women.2 This clinical syndrome is now recognized as a polymicrobial superficial vaginal infection involving a loss of the normal lactobacilli and an overgrowth of anaerobes. While commonly found in increased numbers in women with bacterial vaginosis, Gardnerella vaginalis is not invariably present. G. vaginalis has been reported in from 16 to 42 percent of women with no signs or symptoms of vaginitis.3
Clinical Implications and Morbidity
Bacterial vaginosis is associated with an increased risk of several pathologic conditions, including postoperative infection following hysterectomy4 and postabortion pelvic inflammatory disease5 (Table 1). The risk of plasma cell endometritis in women with bacterial vaginosis has been reported to be 15 times higher than the risk in women without bacterial vaginosis (95 percent confidence interval; range: 2 to 686).6
| Post–induced-abortion pelvic inflammatory disease | |
| Post-hysterectomy vaginal cuff cellulitis | |
| Plasma cell endometritis | |
| In pregnant women: | |
| Amniotic fluid infection | |
| Clinical chorioamnionitis | |
| Postpartum endometritis | |
| Premature rupture of the membranes | |
| Preterm delivery | |
| Low birth weight | |
In pregnant women, bacterial vaginosis is associated with the presence of fetal fibronectin. Women with fetal fibronectin have a 16-fold increase in clinical chorioamnionitis and a sixfold increase in neonatal sepsis.7 The microorganisms found in bacterial vaginosis are also commonly found in the amniotic fluid of women with amniotic fluid infection.8 Women with bacterial vaginosis have an odds ratio of 1.85 (confidence interval: 1.16 to 2.9) for intra-amniotic infection.9 Bacterial vaginosis in women at 23 to 26 weeks of gestation is associated with intra-amniotic fluid infection at term.10
The increased frequency of bacterial vaginosis in unmarried, low-income black women and in women with previous low-birth-weight infants may account for some of the racial gap in preterm births; however, bacterial vaginosis remains a risk factor for preterm low birth weight when variables are adjusted for race.15
The presence of atypical cells on Papanicolaou smear is also more common in women with bacterial vaginosis.16
Diagnosis
Bacterial vaginosis is diagnosed by the presence of three of the clinical and microscopic findings listed in Table 2. The most common symptoms are a thin, homogeneous vaginal discharge and a malodorous, “fishy” smell. The color and amount of discharge varies greatly from patient to patient. The normal pH of vaginal secretions is less than 4.5. In women with bacterial vaginosis, the pH is usually greater than 4.5. When sampling secretions to measure pH, care must be taken to avoid cervical mucous, as cervical mucous normally has a higher pH.
| Homogeneous vaginal discharge (color and amount may vary) |
| Presence of clue cells (greater than 20%) |
| Amine (fishy) odor when potassium hydroxide solution is added to vaginal secretions (commonly called the “whiff test”) |
| Vaginal pH greater than 4.5 |
| Absence of the normal vaginal lactobacilli |
When drops of a 10 percent potassium hydroxide solution are added to the vaginal secretions of a woman with bacterial vaginosis, an amine, or “fishy” odor is released. This test, commonly referred to as a “whiff test,” is positive in women with bacterial vaginosis but can also be positive in patients with Trichomonas infection.
Another diagnostic criterion for bacterial vaginosis is the presence of clue cells on wet mount. Clue cells are vaginal epithelial cells that have a stippled appearance due to adherent coccobacilli (Figure 1). The edges of the cells are obscured and appear fuzzy compared with the normally sharp edges of vaginal epithelial cells. To be significant for bacterial vaginosis, more than 20 percent of the epithelial cells on the wet mount should be clue cells.
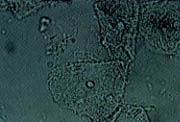
The term vaginosis is used because of the superficial nature of the infection. The wet mount usually does not show the increased number of leukocytes seen in other types of vaginitis. If the wet mount shows increased numbers of leukocytes, a co-infection (e.g., Trichomonas) should be suspected.
An alternative diagnostic criterion utilizes Gram staining of vaginal secretions.17 The loss of lactobacillus morphotypes and increase in Gardnerella and Bacteroides morphotypes and curved gram-variable rods, when combined with the pH, correlates well with Amsel's criteria for diagnosis of bacterial vaginosis.18 Gram stain may not be useful in determining eradication of the infection because of its high proportion of indeterminate results.19 Because the predictive value of a positive culture for G. vaginalis is less than 50 percent, culture is not recommended as a diagnostic tool.20
Some controversy remains over the sexual transmission of bacterial vaginosis. While it occurs more commonly in women with more than one sexual partner, bacterial vaginosis can also occur in women who are not yet sexually active.21 Treatment of male partners has not resulted in improved cure rates or a reduced rate of recurrence.22 Increased rates of infection with Chlamydia trachomatis and Neisseria gonorrhoeae have been reported in women with bacterial vaginosis, but increased rates of syphilis and Trichomonas infection have not been reported.23
Treatment
Treatment regimens for bacterial vaginosis are shown in Table 3. All are safe and effective. The rate of cure for a seven-day course of metronidazole (Flagyl) has been reported to be from 84 to 96 percent; for treatment with oral clindamycin (Cleocin), the cure rate has been reported to be 94 percent24; for metronidazole vaginal gel (Metrogel vaginal), the cure rate has been reported to be 75 percent, and for clindamycin vaginal cream, the cure rate has been estimated to be 86 percent.25 A single 2-g dose of metronidazole may also be effective, but patients given this treatment seem to have a higher rate of recurrence than those given a seven-day course of treatment. Treatment with metronidazole in women undergoing abortion who have bacterial vaginosis reduces the post-abortion risk of pelvic infection to that of women without bacterial vaginosis.26 Oral metronidazole and oral clindamycin have been shown to reduce pregnancy-associated morbidity27–29; topical treatments, however, have not.30,31 Some physicians are hesitant to use metronidazole in women who are pregnant because adequate safety studies have not yet been performed. While issues of mutagenicity remain theoretic, several recent meta-analyses32–34 have reported no association between birth defects and the use of metronidazole during pregnancy.
| Drug | Dosage | Cost (generic)* | |
|---|---|---|---|
| Oral treatment | |||
| Metronidazole (Flagyl) | 500 mg twice daily for seven days | $35.98 ($4.20 to $4.64) | |
| or | |||
| 2 g in a single dose† | 10.29 ($1.20 to $1.32) | ||
| Clindamycin (Cleocin) | 300 mg twice daily for seven days | 35.28 ($27.44 to $28.84‡) | |
| Topical treatment | |||
| Clindamycin 2% vaginal cream | 5 g at bedtime for seven days | 30.23 | |
| Metronidazole vaginal gel (Metrogel vaginal)§ | 5 g twice daily or at bedtime for five days | 26.40 | |
When prescribing metronidazole, the physician should stress to patients the importance of abstinence from all forms of alcohol, as a disulfiram-type reaction can occur. Metronidazole has been shown to interfere with the metabolism of warfarin (Coumadin) and anticonvulsants; consequently, dosages of these agents may need to be reduced. Patients taking barbiturates may require a higher dosage of metronidazole. Clindamycin is also an effective treatment for bacterial vaginosis but is more expensive and is associated with diarrhea and, infrequently, colitis. In nonpregnant women, topical clindamycin 2 percent vaginal cream or metronidazole vaginal gel have rates of cure similar to those for oral treatment. Studies have shown no benefit from treating the sexual partner.
The clinical definition of bacterial vaginosis also includes women without symptoms.35 Researchers from one study29 reported a 50 percent reduction in preterm birth and premature rupture of the membranes associated with bacterial vaginosis after women with the infection had been treated with oral clindamycin. In view of potentially serious sequelae, the question of whether to screen for bacterial vaginosis in pregnancy has been raised.16 Further study is needed to determine if screening for vaginal infections should become a routine part of prenatal care.
Any woman presenting with symptoms of vaginal discharge should be evaluated with a physical examination, wet mount and potassium hydroxide preparation to determine the cause of the discharge so appropriate treatment can be initiated.