
Am Fam Physician. 1998;57(10):2383-2390
This is the second part of a two-part article on office care of premature infants. Part I, on monitoring growth and development, appeared in the last issue (Am Fam Physician 1998;57:2123–30).
See related patient information handout on premature infants, written by the authors of this article.
Medical problems associated with prematurity are frequently complex, and a multidisciplinary approach is often required. Some common problems include the following: (1) anemia, which can be reduced by iron supplementation, (2) cerebral palsy or mental retardation as a result of intraventricular hemorrhage or periventricular leukomalacia, (3) respiratory problems, including bronchopulmonary dysplasia and apnea, (4) visual problems, such as those associated with retinopathy of prematurity, (5) gastroesophageal reflux and (6) surgical problems, including inguinal or umbilical hernia and cryptorchidism. Monitoring of growth and development includes recording the infant's head circumference, weight and length on a growth chart for premature infants. Nutritional status should be assessed at each visit, watching for hyperosmolar problems in infants receiving high-calorie formulas. Consultation with other specialists may be required if abnormalities are identified during follow-up care in the office.
Advancements in the care of premature infants have led to increased survival of these infants, and family physicians frequently provide care to these infants following their discharge from the hospital. Premature infants present many challenges to the family physician. Medical problems originating in the initial weeks of life may require care for months or years. Other conditions may manifest clinically later in infancy or in childhood, necessitating an attitude of continual alertness and attentiveness on the part of the physician. A summary of recommendations for office care is given in Table 1.
| Infant's age | Office data to obtain | Comments |
|---|---|---|
| 1 week after discharge | GROWTH AND DEVELOPMENT | |
| Review prenatal and hospital information; note medications and special formula. Identify risk factors for developmental delay. Record length, weight and head circumference on graph; perform physical examination. | First week is the most stressful; support and encourage mother; be available. | |
| Use growth chart for premature infants. | ||
| NUTRITION | ||
| Review diet (note any hypercaloric formula), feeding pattern and typical 24-hour day. | ||
| SCREENING | ||
| Assess need for community services (WIC, visiting nurse, respiratory therapy, rehabilitation, etc.); immunization status (especially hepatitis B if weight >2,000 g [4 lb, 6 oz]). Check on use of infant car seat. Answer questions. | Follow infant's course weekly until stable growth is documented. Cover after-hours availability. | |
| 2 to 4 weeks after discharge | GROWTH AND DEVELOPMENT | |
| Record length, weight and head circumference on premature growth chart; look for catch-up growth (first head, then weight, then length). | Watch for normal head growth versus hydrocephaly; suspicion of hydrocephaly is higher with history of intraventricular hemorrhage. | |
| NUTRITION | ||
| Review diet, feeding and typical 24-hour day. Add vitamins and iron. Weight-gain goal is 1 oz per day; calorie requirement is usually 100 kcal per kg per day. | Increased calories required in chronically ill infants, such as those with bronchopulmonary dysplasia; up to 200 kcal per kg per day may be required. | |
| SCREENING | ||
| Special testing and consultations (such as audiology, ophthalmology, pneumographs, pulmonary clinic); immunizations: hepatitis B. | May need to check serum theophylline or anticonvulsant levels. Check home adaptation. Follow weekly or biweekly as needed. | |
| 2 to 4 months after discharge | GROWTH AND DEVELOPMENT | |
| Record length, weight and head circumference on premature growth chart; physical examination; developmental testing at regular intervals. | Continued catch-up growth, especially of the head. | |
| NUTRITION | ||
| Review diet, feeding and typical 24-hour day. | Watch for hyperosmolar problems with high-calorie formula. | |
| SCREENING | ||
| Review medications, blood tests, reports from special clinic, and special needs. Immunizations: by chronologic age. | Consider acellular pertussis vaccine; use full doses for immunizations. | |
| 4 to 6 months after discharge | GROWTH AND DEVELOPMENT | |
| Record length, weight and head circumference on premature growth chart; physical examination. | Continue developmental testing at each visit. | |
| NUTRITION | ||
| Review diet, feeding and typical 24-hour day. | In general, solids may be added to diet at four months after due date. | |
| SCREENING | ||
| Review medications, updates from special clinics, blood work needs. Immunizations: hepatitis B, influenza. | Influenza immunization may be given in season to infants >6 months of age, especially those with chronic lung disease, as well as to persons in contact with infant. | |
| 6 to 12 months after discharge | GROWTH AND DEVELOPMENT | |
| Continue recording growth on premature chart through age 2; continue developmental screening. | Catch-up growth is usually achieved by 2 to 3 years of age; some may never catch up. | |
| NUTRITION | ||
| Diet should advance normally unless problems exist, such as swallowing difficulties or reflux. | May switch from formula to whole milk at 1 year of age. | |
| SCREENING | ||
| Monitor medication needs and any blood levels as needed. | Note any clinic reports available. | |
Abnormalities found on screening frequently require consultation with other specialists. Coordination of health care for timely intervention and follow-up is an important role for the family physician, who also must be sensitive to issues of parental support and cost containment. This second part of a two-part article reviews some of the most common medical and surgical problems encountered during the office care of infants born prematurely.
Common Medical Problems
Anemia
Factors that lead to anemia in premature infants include the following: (1) lower iron stores than those in term infants, (2) lower erythropoietin production compared with that in term infants and (3) frequent blood sampling, which can reduce an infant's blood volume by up to 10 percent within a few days of frequent sampling.1 Anemia usually reaches its nadir at one to three months of age, when hemoglobin values of 7 g per dL (70 g per L) are not uncommon in premature infants.
During office visits, signs and symptoms such as tachycardia, tachypnea, pallor, lethargy, poor feeding, poor weight gain and apnea with bradycardia may indicate the presence of anemia. If these signs and symptoms develop, the blood count should be checked. Routine hemoglobin determinations may be considered for infants with hemolytic disease, such as ABO or Rh incompatibility. Although hematocrit levels below 25 percent are often poorly tolerated,2 the need for transfusion should be based on the patient's signs and symptoms rather than on a specific hematocrit level. Infants with large left-to-right shunts usually benefit from a hematocrit level of greater than 40 percent.3
Iron supplementation reduces the level and duration of anemia.4,5 Starting between two weeks and two months of age, iron supplementation in a dosage of 2 to 4 mg per kg per day for 12 to 15 months is recommended.6,7 Ferrous sulfate drops contain 25 mg of elemental iron per mL, and the usual 0.6-mL dose contains 15 mg.
Although it has been postulated that vitamin E reduces hemolysis and is frequently diminished in premature infants, vitamin E supplementation does not affect hemoglobin concentration, reticulocyte count or red blood cell morphology.8 Although erythropoietin is used in some neonatal intensive care units (NICU) in the treatment of anemia of prematurity, it is not routinely recommended.
Developmental Disabilities
One of the greatest fears of parents of a premature infant is mental retardation. Ten to 20 percent of infants with a birth weight under 1,500 g (3 lb, 5 oz) have developmental disabilities.9 Developmental disabilities may range from severe mental retardation and cerebral palsy to a learning disability demonstrable only when the child reaches school age. Developmental disabilities are commonly caused by one of two complications of prematurity: intraventricular hemorrhage or periventricular leukomalacia.
Intraventricular Hemorrhage. This complication occurs in up to 50 percent of infants with a birth weight of 1,500 g (3 lb, 5 oz) or less.10 Grade 1 hemorrhage (blood in the subependymal or germinal matrix) and grade 2 hemorrhage (blood in the ventricles without dilatation) are associated with a 1 to 2 percent risk of cerebral palsy or mental retardation.10 More severe hemorrhages, however, carry significant risks. Grade 3 lesions (blood in the ventricle with ventricular dilatation) are associated with a 30 percent risk of cerebral palsy or mental retardation and a 50 percent risk of some type of developmental disability.10 Grade 4 lesions (intraparenchymal hemorrhage) can carry up to a 70 percent risk of cerebral palsy or mental retardation and a 90 percent risk of developmental disability10 (Figure 1).
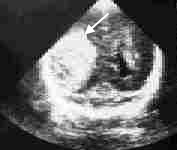
Spastic diplegia is the most common form of cerebral palsy following intraventricular hemorrhage and occurs because of the closer proximity of the lower-limb fibers to the ventricle relative to that of the upper-limb fibers.11 Ventricular dilatation, with or without hydrocephalus, is a potential complication. Catch-up head growth in preterm infants may be difficult to distinguish from hydrocephaly. Cranial ultrasonography should be considered if the infant's head circumference is above the 95th percentile for adjusted age.
Periventricular Leukomalacia. This injury is less common than intraventricular hemorrhage in premature infants, occurring in only 12 percent of infants with a birth weight of less than 1,500 g (3 lb, 5 oz), and it is largely confined to infants weighing less than 1,100 g (2 lb, 6 oz) at birth.10
Periventricular leukomalacia is caused by an ischemic injury to the white matter adjacent to the lateral ventricles. After clearing by phagocytes, the lesion appears ultrasonographically as small cysts (Figures 2 and 3). Mental retardation or cerebral palsy invariably occurs in infants with large bilateral cysts. Even small, focal, unilateral cysts are associated with a 50 to 80 percent risk of mental retardation or cerebral palsy.10,11 Risk factors for the development of periventricular leukomalacia include sepsis, meningitis, hypoxia and seizures. Often, no symptoms are noted in the neonatal period before the infant is discharged from the hospital.

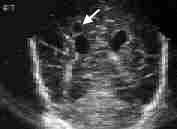
Evidence of any single developmental abnormality in an infant born prematurely signifies the need for a careful search for other abnormalities. Because hypoxic injury is usually a diffuse process, multiple abnormalities are present in many children who incur hypoxic injury. A multidisciplinary team is usually best equipped to perform this evaluation, and consultation with a developmental pediatrician is appropriate.
Early intervention should be undertaken in infants with definite abnormalities. Specific measures, such as nonnutritive sucking (i.e., use of a pacifier) in gavage- or tube-fed infants may be especially helpful in improving growth and development while minimizing oxygen utilization.12
In the absence of intraventricular hemorrhage, periventricular leukomalacia or ventricular dilatation, the incidence of major developmental problems is not increased in premature infants.10 Nearly all infants with normal findings on neurodevelopmental examination at the infant's expected due date continue to develop normally.10 If a child shows no developmental delay during infancy, the risk of mental retardation or cerebral palsy is low. However, continued alertness for developmental problems is warranted because subtle learning disabilities may not be manifested until the school-age years.
Retinopathy of Prematurity
Retinopathy of prematurity occurs in 9 to 24 percent of premature infants born at 32 weeks of gestation or less and causes blindness in 1 to 4 percent.13 Treatment can improve outcome. Cryotherapy of advanced retinopathy of prematurity improves the outcome (a reduction in traction folds and detachments) by 50 percent.14 Retinal laser surgery is also effective and is preferred by many ophthalmologists.
A dilated indirect ophthalmoscopic examination should be performed in all infants with a birth weight of 1,500 g (3 lb, 5 oz) or less, with a gestational age of 28 weeks or less, or who otherwise are thought to be at high risk of retinopathy because of other risk factors such as oxygen therapy and anemia.15 One guideline recommends an initial screening examination at four to six weeks' chronologic age by an ophthalmologist, with follow-up ophthalmologic examinations as needed based on the initial findings.16
Gastroesophageal Reflux
Gastroesophageal reflux is common in premature infants and usually presents as regurgitation. Reflux may also be manifested by apnea, aspiration pneumonia, wheezing or worsening of bronchopulmonary disease. Current use of a nasogastric tube for feedings appears to increase the incidence of reflux.
Even though the risk of sudden infant death syndrome (SIDS) may be increased when infants are lying in the prone position, severe reflux is one reason for placing infants in the prone position for sleeping.18 Alternatively, the infant can be placed on his or her side. Giving the infant smaller, more frequent feedings may be useful, as may therapy with histamine H2-receptor blockers such as ranitidine (Zantac), in a dosage of 2 to 5 mg per kg two times a day, or cimetidine (Tagamet), in a dosage of 2.5 to 5.0 mg per kg four times a day. Thickened feedings appear to help many patients clinically but have not been shown to reduce reflux as measured by a pH probe and may increase the number of coughing spells.17 Metoclopramide (Reglan), in a dosage of 0.1 mg per kg four times a day, and cisapride (Propulsid), in a dosage of 0.2 mg per kg three or four times a day,19 are often used in the treatment of reflux, but there are conflicting studies on the effectiveness of these agents in infants. In addition, metoclopramide carries the small risk of tardive dyskinesia. Surgery may be required in severe cases.
Apnea
Significant apnea, by definition, lasts over 15 seconds and is associated with a drop in oxygen saturation. The heart rate usually drops as well, followed by an acceleration. About 23 percent of premature infants have apnea. About 40 percent of idiopathic apnea episodes in premature infants are central, 50 percent are mixed, and 10 percent are obstructive.20,21 Although most cases of apnea are idiopathic in premature infants, identifiable causes include gastroesophageal reflux, anemia, sepsis, meningitis, upper airway obstruction, hypoxia and bronchospasm.
Periodic breathing is defined as apnea that lasts only five to 10 seconds. This occurs in 40 to 50 percent of premature infants and is thought to be benign.20,21 Although SIDS occurs more frequently in premature infants,22 no clear relationship exists between SIDS and the presence of apnea on testing.
Although apnea monitoring is controversial and unproved as a method of preventing SIDS, many premature infants, both those with and those without documented apnea, are discharged with provisions for at-home apnea monitoring. Criteria for at-home apnea monitoring include the following: infants less than 34 weeks' gestation at birth; significant apnea documented after birth and not associated with a reversible illness; a family history of siblings with SIDS; and bronchopulmonary dysplasia or intraventricular hemorrhage. The home care of infants with apnea primarily consists of home monitoring; the parents should be taught cardiopulmonary resuscitation. Infants nursing in the prone position appear to have less apnea than those fed in the supine position.23
Some newer apnea monitors are capable of recording the infant's respiratory rate, heart rate and oximetry during periods when the alarm is triggered, which helps in determining if the monitor alarm represents a true apnea spell or a false-positive alarm response. Some infants with apnea benefit from theophylline therapy. The usual dosage is 3 to 5 mg per kg every eight to 12 hours to maintain a serum theophylline level of 8 to 12 μg per mL.
Apnea monitoring is commonly continued until the infant is free of symptomatic apnea (apnea associated with a color change or bradycardia) for two months and free of apnea requiring vigorous stimulation for three months. If theophylline is used, the patient can be allowed to “outgrow” the medication when no apnea with bradycardia has been noted for two months.
Chronic Lung Disease
The incidence of bronchopulmonary dysplasia is decreasing as a result of the use of prophylactic surfactant replacement therapy.24 Bronchopulmonary dysplasia occurs primarily in infants with a history of hyaline membrane disease who required supplemental oxygen and mechanical ventilation (Figure 4). A family history of asthma does not increase the risk of bronchopulmonary dysplasia but is associated with more severe disease.25 With severe disease, chest radiography shows cystic lesions. In infants with bronchopulmonary dysplasia who continue to require oxygen after hospital discharge, an oxygen saturation of 94 to 95 percent promotes pulmonary vasodilation and reduces the risk of pulmonary hypertension. Infants receiving oxygen have better weight gain, better development and fewer intercurrent respiratory illnesses than infants with impaired oxygenation who are not treated with supplemental oxygen.
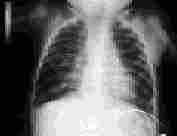
Many infants with bronchopulmonary dysplasia require hypercaloric formulas to promote lung growth and to compensate for the increased work of respiration. Other common treatments include fluid restriction, diuretics and bronchodilators. Although infants with bronchopulmonary dysplasia usually improve and are weaned off oxygen therapy after the first year of life, oxygen may be required during subsequent respiratory illnesses.
Infants with a history of severe neonatal respiratory distress are likely to have persistent respiratory morbidity at one year of age. Those with a history of bronchopulmonary dysplasia also tend to have decreased academic performance.26 Unlike chronic lung disease in adults, lung disease in infants can improve with the growth of new lung tissue. Treatment should be managed in coordination with neonatal consultants.
Seizures
Congenital brain abnormalities, cerebral palsy, ischemia and meningitis are all risk factors for neonatal seizures. In patients with seizures, the risk of developmental problems depends on the underlying etiology of the seizure. Congenital brain abnormalities carry a poor prognosis. Ischemia and meningitis are associated with a moderate risk of developmental problems, whereas hypocalcemia and intraventricular hemorrhage are associated with a relatively good chance for the absence of other developmental problems.11 Patients with seizures should be free of seizures for at least three months before drug withdrawal is considered, and the electroencephalogram should be without epileptiform discharges.
Common Surgical Problems
Circumcision
Circumcision is generally not performed until the infant weighs at least 2,250 g (5 lb). Indications and contraindications for circumcision in infants born prematurely are the same as those in term infants.
Cryptorchidism
Embryologically, the testes begin their descent at the seventh month and by the ninth month, 95 percent are in the scrotum. Premature birth slows this descent, and only 26 percent of testes are intrascrotal by the time the premature infant reaches term age. By one year of age, 94 percent of testes are intrascrotal.27 Lack of descent is associated with an increased risk of testicular cancer later in life.
Surgical consultation should be considered if the testicles have not descended by the time the infant is one year of age. In infants with bilaterally undescended testes, chromosomal analysis in addition to surgical consultation should be a consideration. Unfortunately, orchiopexy may not eliminate the occurrence of subsequent malignancy.28
Inguinal Hernia
Inguinal hernias are more common in male infants and infants with a history of prolonged ventilatory assistance. The incidence in the general population is only 1 percent,29 but it soars to 30 percent in premature infants with a birth weight of 1,000 g (2 lb, 3 oz) or less. Approximately 10 to 50 percent of inguinal hernias are bilateral.30 Because premature infants have very small internal rings, the risk of incarceration is high.
Inguinal hernias are often repaired before hospital discharge if they are noted in the neonatal period. Infants presenting with hernias after discharge should be sent promptly for surgical consultation. If repair under general anesthesia is performed before the infant reaches the age of three months past the due date, inpatient monitoring is advised because of the increased risk of postoperative apnea.
Umbilical Hernia
In contrast to inguinal hernias, umbilical hernias rarely incarcerate. They can safely be managed with observation and usually resolve spontaneously by the time the child reaches three to five years of age. If an umbilical hernia persists beyond then, surgical repair should be considered. Of course, the folk practice of placing a silver dollar or 50-cent piece over the hernia does not increase the chance of closure. This practice should be discouraged through parental education.
Prognosis
Approximately 85 percent of infants with a birth weight under 1,500 g (3 lb, 5 oz) survive.31 Cerebral palsy develops in 5 to 15 percent, and developmental disabilities develop in 25 to 50 percent of these infants.31 Periventricular leukomalacia and intraventricular hemorrhage are major risk factors for these problems.32 Small-for-gestational-age infants who fail to have catch-up growth by eight months of age have a poorer prognosis.33 Infants who reach term age without a serious disorder are likely to have growth and development within the normal range.34
Anemia is common but can be minimized by using iron supplements. Careful physical examinations and monitoring of developmental milestones help identify cerebral palsy or other developmental abnormalities. The presence of developmental abnormalities signals the need for multidisciplinary evaluation. Chronic medical problems can be managed by the family physician along with the neonatologist. Surgical referral for hernias and cryptorchidism must be obtained in a timely fashion. By performing frequent examinations, coordinating the infant's medical care and monitoring the needs of the parents, the family physician can make effective interventions on behalf of the premature infant.