Am Fam Physician. 1998;57(11):2705-2710
The Charcot foot commonly goes unrecognized, particularly in the acute phase, until severe complications occur. Early recognition and diagnosis, immediate immobilization and a lifelong program of preventive care can minimize the morbidity associated with this potentially devastating complication of diabetic neuropathy. If unrecognized or improperly managed, the Charcot foot can have disastrous consequences, including amputation. The acute Charcot foot is usually painless and may mimic cellulitis or deep venous thrombosis. Although the initial radiograph may be normal, making diagnosis difficult, immediate detection and immobilization of the foot are essential in the management of the Charcot foot. A lifelong program of patient education, protective footwear and routine foot care is required to prevent complications such as foot ulceration.
Although initially described in patients with tertiary syphilis, the Charcot foot is now seen mostly in patients with diabetes mellitus. In a recent study,1 9 percent of patients with diabetic neuropathy had Charcot foot. It is a condition of acute or gradual onset and, in its most severe form, causes significant disruption of the bony architecture of the foot. It often results in foot deformities and causes abnormal pressure distribution on the plantar surface, foot ulcers and, in some cases, requires amputation. The exact pathogenesis is unknown, but underlying sensory neuropathy is nearly universal. Arteriovenous shunting due to autonomic neuropathy is also thought to play a role. Repeated unrecognized microtrauma or an identifiable injury may be the inciting factors of Charcot foot. Approximately 50 percent of patients with Charcot foot will remember a precipitating event such as a slip or a trip, or they may have had unrelated surgery on the foot as an antecedent event. In approximately 25 percent of patients, a similar problem ultimately develops on the other foot.
This article focuses on several critical aspects of the diagnosis and management of the Charcot foot that will minimize the risk of limb loss in this relatively common complication. The following is a discussion of six important aspects of the diagnosis and management of the Charcot foot that every primary care clinician should know.
1. The acute Charcot foot can mimic cellulitis or, less commonly, deep venous thrombosis.
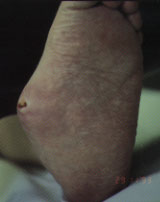
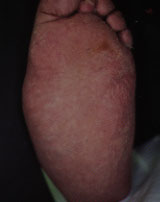
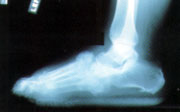
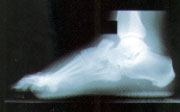
Clinical findings in patients with an acute Charcot process include warmth, erythema and swelling2–6 (Figures 1a and Figures 1b). Pain and tenderness are usually absent because of sensory neuropathy, which is universal and is probably a component of the basic pathogenesis of the Charcot foot. However, since patients with Charcot foot may have some pain if the sensory loss is not complete, the presence of pain does not totally exclude the diagnosis. Such pain is always much less than would be expected for the severity of the clinical and/or radiographic findings. While cellulitis should be considered in any patient with diabetes, missing the diagnosis of Charcot foot can be disastrous since failure to initiate proper treatment of the Charcot foot exacerbates the problem. Inappropriate treatment with antimicrobial therapy and even incision and drainage can lead to unnecessary complications.
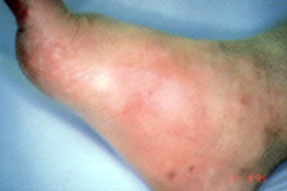
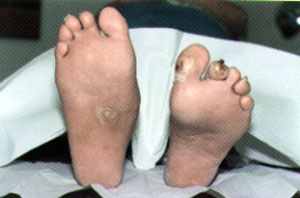
Some clues to the diagnosis of Charcot foot include the following: systemic symptoms and laboratory abnormalities are typically absent in a patient with a Charcot fracture; a radiograph can often differentiate a Charcot foot from infection; the erythema may be more clearly delineated in a patient with cellulitis, and many, if not most, cases of cellulitis have an inciting skin wound. If there is any doubt about the presence of infection, antimicrobial therapy can be prescribed but no weight should be borne on the foot if the diagnosis of a Charcot foot remains a possibility. Although nuclear scans may occasionally be used to distinguish the Charcot foot from infection, these studies are not routinely performed.
Sometimes marked swelling of the foot and lower leg is considered an isolated finding, mimicking deep venous thrombosis. In our experience, the foot itself is more prominently swollen in the Charcot process than in cases of deep venous thrombosis; however, if there is any doubt, a venous duplex scan can be performed to help exclude venous occlusion.
We strongly recommend that the diagnosis of acute Charcot foot be considered in any patient with diabetes and unilateral swelling of the lower extremity and/or foot.
2. The existence of little or no pain can often mislead the patient and the physician.
It is important to remember that both patients and physicians rely on pain as a guide to the severity of a disease process. Minimal pain or the absence of pain (characteristic of a Charcot fracture) can lead patients and physicians to ignore this serious disease.
3. Findings on plain radiographs can be normal in the acute phase of the Charcot foot.
The exact etiology of the Charcot process is unknown. However, bony destruction, fragmentation, joint subluxation and bony remodeling are considered radiographic hallmarks of the disease (Figure 2). These radiographic changes take time to develop, however, and may be absent at the time of presentation. The initial radiographic findings can be normal, making the diagnosis difficult but, if a Charcot foot is strongly suspected from the clinical presentation, treatment should be initiated and serial radiographs should be taken. The proper treatment for a hot, swollen foot in a patient with sensory neuropathy is immobilization. We believe that the best form of immobilization is a total contact cast, when available. The patient with neuropathy presenting to the emergency department with a “sprained ankle” and normal radiographic findings should have the foot immobilized. Subsequent plain radiographs in a patient who was not immobilized usually show evolution of the process (Figure 3).
The patient with an acute Charcot process and normal radiographs whose foot is placed in a cast may not progress. This can create a “Catch-22” situation, since the diagnosis of a Charcot fracture cannot be made definitively until bony changes are demonstrated. A patient without radiographic changes who is placed in a cast can usually be reintroduced to unprotected weight-bearing activity in one to two months, based on the resolution of swelling and local warmth. A nuclear bone scan may occasionally be helpful for early diagnosis in difficult cases.
4. Strict immobilization and protection of the foot (most often in a total contact cast) is the recommended approach to managing the acute Charcot process.
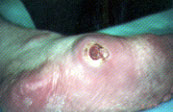
After a diagnosis of Charcot foot has been made, patients should be referred to an orthopedist, a podiatrist or a specialized diabetes foot center for management of the acute phase of the process if the primary care physician is not experienced in the management of the acute Charcot foot. Randomized, prospective trials of different approaches to the management of the Charcot foot have not been performed. However, the standard of care has become strict immobilization of the foot and ankle in an attempt to stabilize and protect the foot. Strict non–weight-bearing measures are recommended by some diabetes foot centers. Currently, in our program, we use the total contact cast (Figure 4), which allows some measure of ambulation for the patient and appears to prevent the progression of deformity. Charcot fractures that are not treated progress, typically leading to marked deformity and skin ulceration over the new bony prominence (Figure 5).
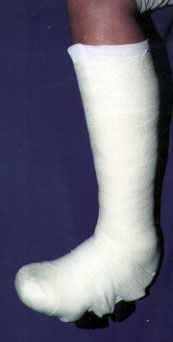
Casts should be replaced approximately every one to two weeks. The foot should be inspected, and cutaneous temperature measurements should be made. Serial plain radiographs should be taken approximately every month during the acute phase. Casts should be kept on until the active phase of the Charcot process is complete, as evidenced by temperature normalization and radiographic stability. Casting usually lasts from three to six months.
5. A careful program of patient education, protective footwear and routine foot care is required to prevent complications such as foot ulceration.
Once the acute Charcot process has subsided and the cast is removed, lifelong protection of the at-risk foot begins. Reactivation of the Charcot process appears to be uncommon, but a foot with significant deformity will always remain at risk for ulceration. Once the cast has been removed, most patients will co-manage their condition with their primary care physicians. The initial post-cast phase usually includes the use of some sort of a brace to protect the foot. In our clinic, we usually use a patellar tendon–bearing brace in addition to custom-molded footwear (Figure 6). The brace can sometimes be eliminated from the regimen after six to 24 months. Thereafter, continued use of custom footwear to protect and support the foot is essential. Foot ulceration, subsequent infection and, ultimately, amputation are common occurrences in the patient with a Charcot foot that has been improperly managed. Patient education and regular foot care by a professional are integral aspects of a lifelong program of foot protection and preservation of skin integrity.

6. Reconstructive surgery is reserved for patients who have recurrent ulceration despite compliance with the previously mentioned regimen.
During the chronic or “cool” phase of the Charcot process, ulceration can be prevented with careful attention to the regimen previously outlined. However, in the uncommon instance of recurrent ulceration despite compliance with a carefully designed regimen, reconstructive surgery to realign the foot and redistribute pressure more favorably is an option that has been gaining popularity in recent years (Figures 7aand 7b). These procedures can be highly successful but, for now, should be reserved for use in selected patients who cannot be managed successfully with aggressive nonsurgical measures.