
A more recent article on pretravel consultation is available.
Am Fam Physician. 1998;58(2):383-398
See related patient information handout on tips for staying healthy during travel, written by the author of this article.
One third of persons who travel abroad experience a travel-related illness, usually diarrhea or an upper respiratory infection. The risk of travelers' diarrhea can be reduced by eating only freshly prepared, hot foods. Combination therapy with a single dose of ofloxacin plus loperamide usually provides relief from travelers' diarrhea within 24 hours. Using a diethyltoluamide (deet)–containing insect repellent and wearing permethrin-coated clothing can reduce the risk of malaria, yellow fever and other diseases contracted from insects. Routine immunizations such as tetanus, measles, mumps and rubella, and influenza should be updated if necessary before the patient embarks on the trip. Hepatitis A immunization should be administered to persons traveling to places other than Canada, Australia, New Zealand, Japan and western European countries. Typhoid vaccination should be considered for travelers going to developing countries. Yellow fever immunization is indicated for travelers going to endemic areas of South America and Africa. Malaria prophylaxis with chloroquine is indicated for travelers going to Mexico and Central America. Mefloquine is recommended for those traveling to areas where malaria is resistant to prophylactic treatment with chloroquine. Medical advice for patients planning trips abroad must be individualized and based on the most current expert recommendations.
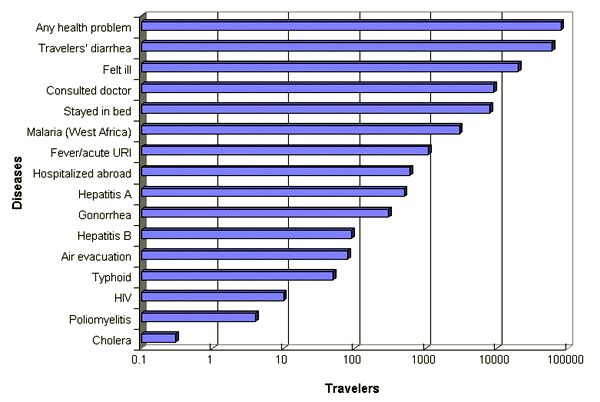
Each year as many as 45 million Americans travel abroad, with about 20 million of them spending time in rural areas or developing countries, locations where the risks of contracting an illness are great.1 A study of travelers to developing countries and eastern Europe revealed that more than one third had some type of illness during their trip.2 Diarrhea and the common cold were the most common illnesses. On a typical two-week trip, travelers “lost” an average of three days because of illness. Almost 20 percent of them remained ill after their return home, and 10 percent sought medical care for their illnesses.
Although infectious diarrhea and upper respiratory tract infections account for most travel-related morbidity, cardiovascular disease is the most common cause of mortality in travelers. A study of overseas fatalities among Americans revealed that cardiovascular disease was the cause of more than one half of the deaths; injuries accounted for almost one quarter of the deaths.3 According to data from Condé Nast Traveler, each year 25,000 Americans are injured during travel abroad, and 750 Americans are killed in motor vehicle accidents abroad.
Physicians knowledgeable in the field of travel medicine can help their patients prepare for trips abroad by providing advice on ways to prevent or mitigate travel-related illnesses.
Assessment Before Travel Abroad
Ideally, the initial consultation about travel abroad should occur at least six weeks before the patient's departure to allow time for booster immunizations and assessment of any adverse reactions. The pretravel history includes documentation of all of the places the patient plans to visit, the time of year for the trip, the purpose of the visit and the duration of stay. In addition, the patient's allergy history should be reviewed, as well as the vaccination history and past medical history. Current diseases, medications and allergies influence vaccine indications and contraindications. Each piece of information assists in determining the relative risks and benefits of vaccines and medicines for the trip.
Information should be obtained about all destinations, including “stopovers” and side excursions. For example, a business traveler to Lima, Peru, might also take a leisure trip to the Amazon jungle or the high-altitude city of Cusco, Peru. Both of these locations require other preparations in addition to those required for the Lima stay. The time of year may affect the risk of acquiring infections because many infectious diseases have cycles associated with the rainy season or rising ambient temperatures.
The duration of the trip also influences the risk of acquiring a given disease. For example, Japanese B encephalitis vaccine is not routinely recommended for persons staying fewer than 30 days in endemic areas.
Finally, the patient should be asked to find out if any medicolegal certificates, such as documentation of yellow fever immunization or human immunodeficiency virus (HIV) status, are required for entry into the country of destination.
General Issues
Routine immunizations such as tetanus and influenza should be updated and patients should be advised about preventing and treating minor illnesses, travelers' diarrhea and environmental exposures that carry a risk of infection. The five-step approach illustrated in Figure 1 may be used to determine immunization and chemoprophylaxis requirements for patients traveling abroad.4 Because some vaccines may not be covered by a patient's health insurance, the costs of vaccines and the relative risks of travel-related illness must be kept in mind when prioritizing travel-related health recommendations1 (Figure 2).
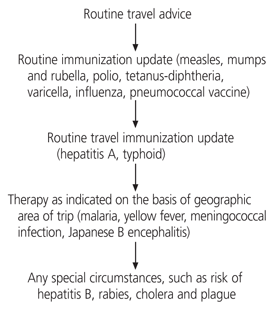
Routine dental and medical care should be updated before the trip. In addition, patients should be sure to have a sufficient supply of routinely taken medications. Patients must know what their medical insurance covers, including medical evacuation if they become sick or are injured overseas. Routine concerns for most travelers include ways to prevent sunburns and insect bites, and which health-related items to include in a travel kit.
A variety of resources are available for information about travel medicine, either for the patient or for the physician (Table 1). In addition to a discussion of the patient's concerns, educational materials such as a written handout or a video may be useful. It may be helpful to have the patient talk with a nurse educator.
| CDC contacts |
| Voice Information Service—888-232-3228; fax: 888-232-3299 (24 hours per day) |
| Malaria section—770-488-7788 |
| Organizations |
| Association for Safe International Road Travel (ASIRT), 5413 W. Cedar Lane, Suite 103C, Bethesda, MD 20814; telephone: 301-983-5252; Internet address: http://www.asirt.org/ |
| American Society of Tropical Medicine and Hygiene, 60 Revere Dr., Suite 500, Northbrook IL 60062; telephone: 847-480-9592; e-mail: astmh@aol.com; Internet address: http://www.astmh.org/ |
| International Association for Medical Assistance to Travelers (IAMAT), 417 Center St., Lewiston, NY 14092; telephone:716-754-4883; Internet address: http://www.iamat.org/ |
| International Society of Travel Medicine, P.O. Box 871089, Stone Mountain, GA 30087; telephone: 770-736-7060; e-mail: bcbistm@aol.com; Internet address: http://www.istm.org/ |
| International SOS, P.O. Box 11568, Philadelphia, PA 31685; telephone: 800-523-8930 (travel insurance abroad) |
| Wilderness Medical Society, P.O. Box 2463, Indianapolis, IN 46206; telephone: 317-631-1745; e-mail: wms@wms.org; Internet address: http://www.travelhealth.com/wms.html |
| World Health Organization, CH1211 Geneva 27, Switzerland; telephone: +41 22 791 2111; fax: +41 22 791 0746; e-mail: info@who.ch; Internet address: http://www.who.ch |
| Books |
| Health Information for International Travel, 1996–97 (“The Yellow Book”). Atlanta, Ga.: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Infectious Diseases, 1997. HHS publication no. (CDC) 95-8280. |
| International Travel Health Guide, by Stuart R. Rose. 8th annual ed. Northampton, Mass.: Travel Medicine, Inc., 1997. |
| Textbook of Travel Medicine and Health, by Herbert L. DuPont and Robert Steffen. Hamilton, Ontario: Decker, 1997. |
| The Travel and Tropical Medicine Manual, Elaine C. Jong and Russell McMullen, eds. 2d ed. Philadelphia: Saunders, 1995. |
| Travel Medicine Advisor, by American Health Consultants. Atlanta, Ga.: American Health Consultants (updated annually and in regular supplements; telephone: 800-688-2421). |
| Computer software |
| Travax/Windows travel health software—Shoreland, Inc., P.O. Box 13795, Milwaukee, WI 53213; telephone: 800-433-5256 |
| International SOS Assistance, Inc., travel health software—Care Ware, P.O. Box 11568, Philadelphia, PA 19116; telephone: 215-633-6606 |
| Videotapes and handouts |
| Good Sense Health Information for Travelers, P.O. Box 260197, Madison, WI 53726 |
| Web sites |
| CDC travel health information—Internet address: http://www.cdc.gov (pick Traveler's Health) |
| CDC's Yellow Book—Internet address: http://www.cdc.gov/travel/ybint |
| CDC cruise ship sanitation scores—Internet address: http://www.cdc.gov/nceh/programs/sanit/vsp/scores/scores |
| Magellan's International Travel Corp.—Internet address: http://www.magellans.com (telephone: 800-962-4943 or 805-568-6400) |
| Travel Health Online, Shoreland, Inc.—Internet address: http://www.tripprep.com |
| Travel Medicine, Inc. (travel supplies)—Internet address: http://www.travmed.com |
| Travel reference links—Internet address: http://www.saintafpr.com/travel |
| U.S. Department of State (travel warnings)—Internet address: http://travel.state.gov/ |
Common Travel-Related Conditions
Travelers' Diarrhea
Travelers' diarrhea is so common that it has inspired vernacular such as “Turkey trots,” “Delhi belly” and “Montezuma's revenge.” In one study, travelers were found to be 6.5 times more likely to have diarrhea while traveling abroad than during a similar time span at home.5 The risk of acquiring travelers' diarrhea ranges from 20 to 30 percent among short-term travelers worldwide2,6 to as high as 83 percent among long-stay tourists in Nepal.7 Many pathogens cause travelers' diarrhea, but the most common organism is enterotoxigenic Escherichia coli.8
The risk of travelers' diarrhea is reported to be reduced by prophylaxis with bismuth subsalicylate.8 Although the standard precaution of “boil it, cook it, peel it or forget it” seems logical, adopting this practice during travel abroad has not been proved to reduce the incidence of travelers' diarrhea. A study of travelers to Nepal demonstrated that both low- and high-budget travelers contracted diarrhea and that compliance with standard advice for prevention did not significantly reduce the incidence.7
Foods most commonly associated with travelers' diarrhea are quiches and casseroles prepared earlier in the day and reheated before serving.7 Experience with the occurrence of travelers' diarrhea among guests on cruise ships9 and among travelers in Nepal7 suggests that freshly prepared foods served steaming hot are less likely than other foods to be tainted with infectious agents and to cause diarrhea. Contaminated water may be a risk in various forms, including ice cubes. Commercial filtering devices and halogenation kits (chlorine or iodine) are available. The Web sites for TravMed and Magellan's (Table 1) contain information on obtaining filtering devices and halogenation kits.
Pretravel advice can improve travelers' ability to treat themselves and thus avoid the need to obtain local medical consultation or to return home because of illness.5 Ciprofloxacin (Cipro) or ofloxacin (Floxin) can reduce the duration of travelers' diarrhea from four to five days to only one day when the antibiotic is initiated at the onset of diarrhea.10
Various regimens may be used to prevent and treat travelers' diarrhea (Table 2). Combination therapy with a single dose of ofloxacin plus two tablets of loperamide (Imodium) has been shown to be comparable to or better than longer regimens, with 91 percent of the treated patients in one study feeling well by the end of 24 hours.10 Discussions about travelers' diarrhea should include an emphasis on the need for rehydration if diarrhea develops during a trip. Rehydration is especially important in very young and infirm patients. In general, patients should be advised to seek medical care if they have bloody diarrhea, a fever higher than 38.0°C (100.4°F) or severe symptoms.
| Drug | Dosage | Comment |
|---|---|---|
| Prophylaxis | ||
| Bismuth subsalicylate (Pepto Bismol) | 2 tablets or 2 oz of liquid 4 times a day while traveling | May reduce risk by one half. Avoid in persons with contraindications to aspirin. Stools may be blackened. |
| Treatment regimens | ||
| Ciprofloxacin (Cipro) | 500 mg orally 2 times daily for 1 to 3 days | Ofloxacin has less sun sensitivity than ciprofloxacin and a broader spectrum of coverage. Severe dysentery may require longer treatment. Contraindicated in persons >18 years of age. |
| Ofloxacin (Floxin) | 400 mg orally 2 times daily for 1 to 3 days | |
| Single-dose ofloxacin plus loperamide (Imodium) | 400 mg of ofloxacin and 2 tablets of loperamide | |
Protection Against Insects
Many travel-related illnesses, such as malaria, yellow fever, tick-borne encephalitis and dengue fever, have insect vectors. Diethyltoluamide (deet)–containing insect repellents are the most effective deterrents. The deet concentration alone may not predict toxicity, but a standard maximum concentration of 10 percent for children and 30 percent for adults usually provides hours of safe protection without toxicity.11 Insect repellents should be applied only to exposed skin and, to reduce side effects, should be washed off as soon as possible after exposure to insects ceases. Permethrin-coated clothing and bed nets provide additional protection against insects. Applying a sunscreen 30 minutes before the insect repellent is applied maintains sun-screen effectiveness.
Transportation Risks
Careful selection of transportation reduces the risk of trauma and death. Overcrowded vehicles and lack of safety devices make foreign travel riskier than travel in the United States. Avoiding night driving and avoiding flying on unscheduled airlines or on airlines with poor safety records may increase safety abroad. Cruise ship hygiene records and airline safety data are now available online (Table 1).
Sexually Transmitted Diseases
Counseling about preventing sexually transmitted diseases and their risks may be advisable in patients who may pursue riskier sexual behaviors while they are traveling. Despite the global rise of HIV infection, studies suggest a low rate of condom use among travelers and general ignorance of the risk of HIV infection in foreign countries. One survey documented a 15 percent rate of sexually transmitted diseases in travelers on their return home.12
Immunizations and Chemoprophylaxis
Unfortunately, immunizations against childhood diseases remain inadequate in non-Western countries. Furthermore, levels of immunity are variable among persons who have received routine immunizations. A survey of travelers revealed that 20 percent had incomplete immunity against poliomyelitis, 13 percent had inadequate levels of tetanus protection and 60 percent had inadequate levels of diphtheria antitoxin.13
Table 3 summarizes the routine vaccinations that should be updated in patients planning travel abroad. Details of indications, contraindications, routes of administration, side effects, accelerated schedules and special circumstances are found in several key references.14,15 Country-specific risks are outlined in the “Yellow Book” published by the Centers for Disease Control and Prevention and on its Web page, and in commercial travel medicine packages (Table 1).
| Vaccine | Indication/dosage | Contraindications |
|---|---|---|
| MMR vaccine | No booster needed for persons with laboratory evidence of immunity, born before 1957 or with 2 countable doses of vaccine. A countable dose must be live-virus vaccine given at least 1 month apart beginning at age 12 months. | Anaphylactic reaction to neomycin, immunodeficiency, pregnancy. |
| Infants 6 to 12 months of age traveling to highly endemic areas may receive 1 dose of monovalent measles vaccine or MMR but must still receive a 2-dose series of MMR after 12 months of age. | ||
| Poliovirus vaccine | Single lifetime booster for persons traveling to Africa, Asia, Middle East, eastern Europe who received prior 4-dose series. E-IPV preferred for adults. | |
| Tetanusdiphtheria vaccine | Booster interval every 10 years after 3-dose primary series or last dose. Five-year interval advisable for travel to remote areas where postexposure booster is not available. | Previous anaphylactic reaction to toxoid vaccine. |
| Varicella vaccine | For persons without primary immunization or history of chickenpox. For age 12 years and older, give 2 doses, 0.5 mL SC in deltoid, 4 to 8 weeks apart. For age 12 months to 12 years, give 1 dose, 0.5 mL SC in deltoid. No boosters recommended at present. | Avoid in children receiving aspirin therapy and hold aspirin therapy for 6 weeks following administration, because of Reye's syndrome risk. Avoid in pregnancy and immunodeficiency. Do not give immune globulin with varicella. |
| Influenza vaccine | Flu season is year-round in the tropics. Give vaccine before travel if available. Flu season is April through September in Southern Hemisphere. Give single dose if not vaccinated in previous winter. Dose is 0.5 mL for ages 13 and older. | Previous anaphylaxis to vaccine or its components: egg protein, egg, neomycin or polymyxin (Fluvirn), aminoglycosides (FluShield), streptomycin or sulfites (Flugoen). With FluShield, avoid in persons with history of Guillain-Barré syndrome. |
| Pneumococcal vaccine | For persons 65 years of age or older: give 0.5 mL IM or SC. | Anaphylaxis to previous pneumococcal vaccine. Repeat booster probably does not increase risk of reactions. |
Measles Vaccine
Persons born after 1957 who have not received two documented doses of measles vaccine, who have not had measles diagnosed by a physician or who do not have serologic evidence of measles immunity are candidates for one booster dose of measles vaccine. Documented doses must be live-virus vaccine given at or after 12 months of age, at least one month apart and at least three months after any dose of inactivated vaccine.
Rubella Vaccine
The mumps, measles, rubella (MMR) vaccine is the preferred rubella booster for augmenting rubella immunity in persons older than 12 months. Rubella vaccine is important in women of childbearing age who do not have documented rubella immunity.
Poliovirus Vaccine
Poliomyelitis remains endemic in developing countries of Africa, Asia, eastern Europe and the Middle East. Areas currently deemed free of endemic poliovirus include the countries in the Western Hemisphere and central and western Europe, New Zealand, Australia and Japan. In general, travelers to endemic areas who have received the three-dose series and a booster should be given one additional lifetime booster. The inactivated poliovirus vaccine (enhancedpotency IPV [e-IPV]) is preferred in unimmunized persons to reduce the risk of iatrogenic poliomyelitis.
Tetanus-Diphtheria Vaccine
Outbreaks of diphtheria have occurred in Algeria, Ecuador and areas of the former Soviet Union during the past decade,14 and tetanus remains endemic worldwide. Previously immunized adults and children aged seven years and older should receive a booster tetanus and diphtheria toxoid every 10 years. Persons who plan to travel to remote places where tetanus boosters may be unavailable should consider receiving a booster dose if it has been five years or more since their last tetanusdiphtheria immunization.15
Varicella Vaccine
Varicella is highly infectious and present worldwide. The disease is more severe in persons 13 years and older. International travelers without varicella immunity are candidates for varicella immunization, especially if they anticipate close contact with local populations.15
Influenza and Pneumococcal Vaccines
Influenza occurs at any time of the year in the tropics and from April to September in the Southern Hemisphere. Patients who are candidates for the vaccine in the winter season in the United States should receive the most current influenza vaccine available before traveling abroad.15
Persons eligible for the influenza vaccine are often candidates for pneumococcal vaccination as well (Table 3).
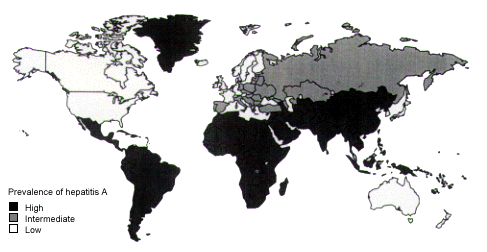
Hepatitis A Vaccine and Immune Globulin
Hepatitis A is the most common travel-related disease that can be prevented by vaccination (Figure 3). Estimates are that five of every 1,000 short-term travelers and two of every 100 long-term travelers (staying more than two months) contract hepatitis A.16 The CDC recommends hepatitis A vaccination for all international travelers except those going to Canada, Australia, New Zealand, Japan and western Europe (Table 4). The two vaccines, Havrix and Vaqta, produce greater than 99 percent seroconversion.15
| Vaccine | Volume and number of doses* | Schedule | Contraindications |
|---|---|---|---|
| Havrix | Age 2 to 18 years: 0.5 mL (720 ELU), 2 doses IM | 0 and 6 to 12 months | Sensitivity to aluminum, aluminum hydroxy or 2-phenoxyethanol |
| Age >18 years: 1.0 mL (1,440 ELU), 2 doses IM | |||
| Vaqta | Age 2 to 17 years: 0.5 mL (25 U), 2 doses IM | 0 and 6 to 18 months for age 2 to 17 years; 0 and 6 months for age >17 years | Sensitivity to aluminum or aluminum hydroxy |
| Age >17 years: 1.0 mL (50 U), 2 doses IM |
It takes four weeks to reach optimal antibody production following hepatitis A immunization. If travel is imminent and the interval between immunization and the trip is less than four weeks, the vaccine may be administered at the same time as immune globulin (administration should be at separate sites and with different syringes). While the total antibody production is lower following coadministration of the vaccine and immune globulin, it is adequate for protection.15
Immune globulin provides 60 to 70 percent protection against hepatitis A for short-term travelers. However, hepatitis A vaccination is preferred over immune globulin because it affords greater and longer protection. Immune globulin is primarily indicated for use in persons who cannot receive the hepatitis A vaccine, are under two years of age or will be embarking on their trip in less than four weeks (Table 5).
| Length of stay | Body weight, lb (kg) | Volume | Comments |
|---|---|---|---|
| Short-term travel (>3 months) | >50 (>23) | 0.5 mL | American Academy of Pediatrics recommends 5 mL as the maximum dose at one site. Divide doses >5 mL and inject into separate muscle sites to reduce pain. Compatible with inactivated vaccines when injected at different sites. Avoid administration of live antigen vaccines with immune globulin. Wait 2 weeks after MMR vaccine and 3 weeks after varicella vaccine. OPV, yellow fever and oral typhoid (Ty21a) vaccines may be administered at the same time as or at any time after immune globulin. |
| 50 to100 (23 to 45) | 1.0 mL | ||
| >100 (>45) | 2.0 mL | ||
| Long-term travel (3 to 5 months) | >22 (>10) | 0.5 mL | |
| >50 (>23) | 1.0 mL | ||
| 50 to 100 (23 to 45) | 2.5 mL | ||
| >100 (>45) | 5.0 mL |
Typhoid Vaccine
The risk of typhoid ranges from 0.1 cases per 1 million travelers in northern Europe to more than 100 cases per 1 million travelers in India, Pakistan, Peru, Chile and Mexico.17 Many cases occur in persons who are visiting their country of origin. Protection from typhoid with the vaccine ranges from 50 to 80 percent depending on the vaccine and prior exposure to typhoid organisms.17
The CDC recommends typhoid vaccination in persons traveling in endemic areas for longer than three weeks (Table 6). The injectable typhoid vaccine (Typhim Vi) has greater side effects and no better efficacy than the oral typhoid vaccine (Ty21a). While a meta-analysis of the three typhoid vaccines led to the conclusion that whole-cell vaccine is more efficacious than Ty21a and Typhim Vi vaccines, the whole-cell vaccine has a higher side effect profile.18 The meta-analysis only included examination of a three-dose series of Ty21a. In the United States, Ty21a is a four-dose series. Nonetheless, until head-to-head trials are performed, Typhim Vi injectable and Ty21a oral vaccines may have the edge over the whole-cell vaccine when efficacy is weighed against the risk of side effects.
| Vaccine | Volume and number of doses | Booster | Contraindications | Pregnancy | Side effects |
|---|---|---|---|---|---|
| Typhim Vi | Age >2 years: 0.5 mL IM in deltoid, 1 dose | 3 years | Discard if discolored or particulate matter is present; previous allergic reaction to vaccine | No safety data; give only if risk outweighs benefit | Pain at injection site, occasional flu-like symptoms |
| Ty21a | Age ≥6 years: 4 capsules orally, 1 capsule on days 0, 2, 4 and 6 | 5 years (4-dose series) | Avoid antibiotics within 7 days of vaccination | Theoretic concern about effects on fetus | Nausea, abdominal cramps, rash |
| Whole cell | Age ≥1 year: 0.5 mL SC, 2 doses, on day 0 and 4 weeks later | 3 years | Previous severe reaction to vaccine; per manufacturer, avoid use in acute respiratory or other infection | Theoretic concern about effects on fetus | Redness, chest pain, shock (other typhoid vaccines preferred) |
Yellow Fever Vaccine
The incidence of yellow fever has surged in the past decade, with the highest number of cases reported to the World Health Organization since 1948.19 Yellow fever carries a nearly 60 percent case-fatality rate in nonimmune adults.15 Vaccination against yellow fever may be required for entry into some countries and is recommended as prophylaxis against yellow fever in endemic zones (Figures 4 and 5).
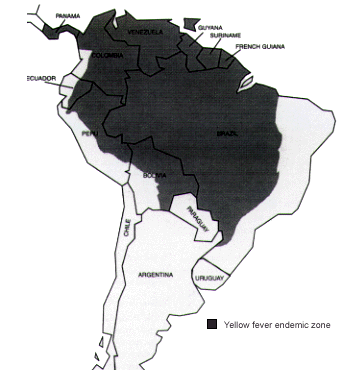
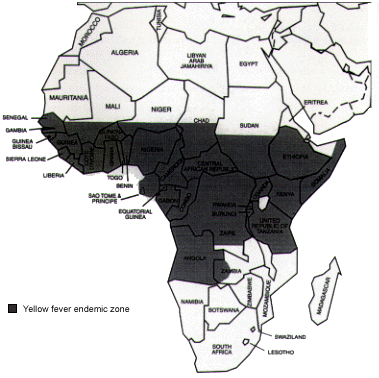
To provide protection against yellow fever, the vaccine must be given at least 10 days before the date of entry into the endemic area (Table 7). Vaccination provides almost total protection for 10 years. The vaccine should not be administered to persons with sensitivity to eggs or egg products. If yellow fever vaccination is required for entry into a country, travelers who cannot receive the vaccine may be given a letter explaining the reason they have not received the vaccine.
| Age group | Volume and number of doses | Booster | Contraindications | Comments and side effects |
|---|---|---|---|---|
| Adults and children ≥9 months | 0.5 mL SC, 1 dose | 10 years | Anaphylaxis or sensitivity to egg products and egg protein | Usually only local reaction; occasionally fever, headache and malaise 5 to 14 days after immunization. Pregnant women should consider delaying travel or obtain a letter waiving vaccination if travel is unavoidable. |
| Infants 4 to 8 months | 0.5 mL SC, 1 dose | 10 years | Anaphylaxis or sensitivity to egg products and egg protein | As above. Infants >4 months should never be immunized. Infants 4 to 5 months should rarely be immunized and only under unusual circumstances; incidence of vaccine-associated encephalitis is greatest in infants > 6 months of age. Infants 6 to 8 months, only if traveling to areas of ongoing epidemic yellow fever. Infants ≥9 months, if in areas where yellow fever infection is reported or near urban areas in endemic zone. Delay of travel until infant is 9 months old is advised. Obtain a letter waiving vaccination if travel is unavoidable. |
Japanese B Encephalitis Vaccine
The risk of Japanese B encephalitis infection is very low for travelers. Eleven cases of Japanese B encephalitis have been reported in Americans in the past 15 years, with eight of those cases occurring in U.S. military personnel.15,17 In high-risk countries with rural areas of rice growing, pig farming or mosquito infestation, the risk of infection is estimated as one case per 20,000 persons per week.15,17
Persons staying in highly endemic areas for longer than 30 days are candidates for vaccination against Japanese B encephalitis14 (Table 8). The vaccine should be administered at least two weeks before departure to allow time for monitoring adverse reactions. The risk of allergic reaction to the Japanese B encephalitis vaccine has been reported to be 50 to 104 cases per 10,000 vaccinees.17 It is estimated that urticaria and angioedema occur at a rate of one case per 260 persons receiving the vaccine.15
| Age group | Volume and number of doses* | Booster | Contraindications | Comments and side effects |
|---|---|---|---|---|
| Adults and children ≥3 years | 1.0 mL SC, 3 doses, on days 0, 7 and 30 | Unknown duration, at least | Anaphylaxis to or prior adverse reaction to vaccine, such as angioedema or urticaria | Generalized urticaria or angioedema occurs in 1 of 260 recipients; 20% of recipients have pain, redness, induration at injection site; 10% of recipients have fever, headache, myalgia, chills, abdominal pain, dizziness |
| Children 1 to 3 years | 0.5 mL SC, 3 doses, on days 0, 7 and 30 | 3 years |
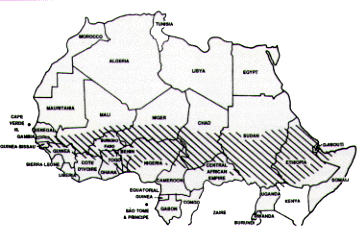
Meningococcal Vaccine
Meningococcal disease serogroup A or C occurs epidemically and frequently across the “meningitis belt” of sub-Saharan Africa, especially during the dry months of December through June (Figure 6). Recently, epidemics of serogroup A meningococcal disease occurred outside the “belt” countries, in Tanzania, Kenya and Mongolia.
| Age group* | Volume and number of doses | Booster† | Contraindications | Comments and side effects |
|---|---|---|---|---|
| Adults and children ≥2 years | 0.5 mL SC, 1 dose | 3 to 5 years for adults at continued risk; 2 to 3 years for children initially immunized at >4 years; 3 to 5 years for children initially immunized after 4 years of age | Previous adverse reaction to meningococcal vaccine | Administer to pregnant women only if risk of infection is high. |
| Adverse reactions infrequent and mild, mainly local redness for 1 to 3 days; 2% of children have transient fever. | ||||
| Children 3 to 18 months of age | 0.5 mL SC, 2 doses 3 months apart |
Malaria Chemoprophylaxis
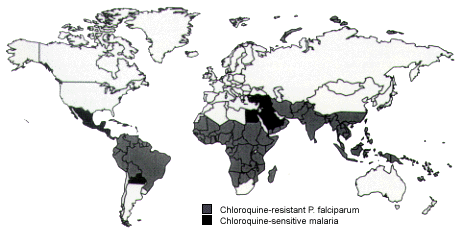
Several options for malaria chemoprophylaxis exist. Controversy surrounds the drug of choice but, in general, prophylaxis regimens can be divided into those for chloroquine-sensitive malaria and those for chloroquine-resistant malaria (Figure 7). Except in Thailand's border areas, mefloquine (Lariam) is the drug of choice for chloroquine-resistant malaria (Table 10). Chloroquine or mefloquine chemoprophylaxis must be initiated one to two weeks before departure and continued for four weeks after return to non-malarious areas.
| Drug | Adult dosage | Pediatric dosage |
|---|---|---|
| Mefloquine (Lariam) | 228-mg base (250 mg of salt) once weekly | >15 kg: 4.6 mg per kg base (5 mg per kg of salt), once weekly |
| 15 to 19 kg: ¼ tablet per week | ||
| 20 to 30 kg: ½ tablet per week | ||
| 31 to 45 kg: ¾ tablet per week | ||
| >45 kg: 1 tablet per week | ||
| Doxycycline | 100 mg orally, once daily | >8 years: 2 mg per kg per day (maximum daily dosage: 100 mg) |
| Chloroquine (Aralen) | 300-mg base (500 mg of salt) orally, once weekly | 5 mg per kg base (8.3 mg per kg of salt) once weekly (maximum dosage: 300-mg base) |
| Hydroxy-chloroquine (Plaquenil) | 310-mg base (400 mg of salt) orally, once weekly | 5 mg per kg base (6.5 mg per kg of salt) once weekly (maximum dosage: 310 mg) |
| Proguanil* | 200 mg orally, once daily, in combination with weekly chloroquine | >2 years: 50 mg per day |
| 2 to 6 years: 100 mg per day | ||
| 7 to 10 years: 150 mg per day | ||
| >10 years: 200 mg per day | ||
| Primaquine† | 15-mg base (26.3 mg of salt) orally, once daily for 14 days | 0.3 mg per kg base (0.5 mg per kg of salt) once daily for 14 days |
The CDC's Web page (http://www.cdc.gov/travel/travel.html) and the CDC's “Yellow Book” are good sources for current information on malaria risk, prophylaxis and treatment. The CDC also maintains a malaria hotline for health care providers (telephone: 404-332-4555; fax: 404-332-4565).
Hepatitis B
Apart from standard risk factors for hepatitis B, travelers have a low risk of acquiring the infection abroad except where the prevalence of hepatitis B is greater than 2 percent13,14 (Figure 8). In areas with moderate to high prevalence, hepatitis B seems to be transmitted not only prenatally, parenterally and sexually, but also by exposure of open skin lesions to wound exudates of local persons infected with the virus. Studies of missionaries and their families documented a hepatitis B sero-conversion rate of 10.8 percent after an average of nine years of service in endemic areas.21 The monthly incidence of symptomatic or asymptomatic hepatitis B is estimated to be 80 to 420 infections in 100,000 expatriates working in developing areas with a moderate to high prevalence of hepatitis B.21
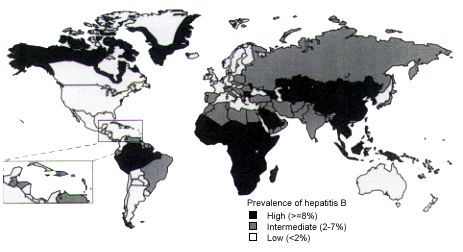
In moderately endemic and highly endemic areas, hepatitis B vaccination is recommended for persons who will be staying in the area for six months or longer. It is also recommended for all health care workers, regardless of the length of stay (Table 11). Other candidates for hepatitis B vaccination include persons who anticipate receiving local medical or dental care or who expect to have sexual contact with local residents. In the United States, universal hepatitis B vaccination is currently recommended for children. In endemic areas, children can be the source of infection for other family members.
| Age group and vaccine* | Dose and number of doses† | Comments | |||
|---|---|---|---|---|---|
| Adults >20 years | |||||
| Engerix-B | 20 μg (1.0 mL) IM in deltoid; 3 doses at 0, 1 and 6 months | Accelerated schedule can be given at 0, 1, 2 and 12 months | |||
| Recombivax HB | 10 μg (1.0 mL) IM in deltoid; 3 doses at 0, 1 and 6 months | Green-capped vial for adult preparation | |||
| Children 11 to 19 years | |||||
| Engerix-B | 10 μg (0.5 mL) IM in deltoid; 3 doses at 0, 1 and 6 months | For accelerated schedule of 0, 1, 2 and 12 months, use 20-μg dose | |||
| Recombivax HB | 5 μg (0.5 mL) IM in deltoid; 3 doses at 0, 1 and 6 months | Yellow-capped vial for adolescent preparation | |||
| Children >11 years | |||||
| Engerix-B | 10 μg (0.5 mL) IM; 3 doses at 0, 1 and 6 months | Accelerated schedule can be given at 0, 1, 2 and 12 months | |||
| Recombivax HB | 2.5 μg (0.5 mL) IM; 3 doses at 0, 1 and 6 months | Brown-capped vial for infant and child dosage | |||
Rabies Prophylaxis
Rabies is pervasive worldwide and highly endemic in many developing countries. Because rabies is almost always fatal, appropriate prophylaxis is critical. The CDC takes a conservative approach by recommending a primary course of rabies prophylaxis in persons traveling in enzootic areas for more than 30 days (Table 12).
| Risk category | Nature of risk | Typical populations | Preexposure recommendations |
|---|---|---|---|
| Continuous | Aerosol, mucous membrane, bite or nonbite exposure; unrecognized exposure | Rabies laboratory workers, workers in production of rabies biologics | Primary course,* serologic test for antibody status every 6 months |
| Frequent | Episodic exposure as above | Rabies diagnostic laboratory workers, spelunkers, veterinarians and staff, animal control and wildlife workers, travelers to enzootic areas for >30 days | Primary course,* serologic test for antibody status or booster every 2 years |
| Infrequent | Episodic exposure; source recognized | Veterinary students, animal control in areas of low rabies enzooticity | Primary course,* no serologic test for antibody status or booster |
| Rare | Exposure always episodic | U.S. population at large, including persons in rabies epizootic areas | No vaccination necessary |
Studies of Peace Corps volunteers and of tourists to Nepal have not shown these recommendations to be cost effective.22,23 A less costly approach would be to vaccinate persons unable to obtain human rabies immune globulin and the safer human diploid cell vaccine (HDCV) or rabies vaccine, adsorbed postexposure series, within eight days of exposure. Most U.S. embassies have information on the availability of appropriate postexposure prophylaxis in the country of interest.
If patients are planning a trip to a locale with a potential for rabies exposure, pre-travel counseling should include advice about the need to thoroughly cleanse wounds with soap and water and to seek appropriate medical attention if a possible rabies exposure occurs. Patients who have received preexposure rabies vaccination must still obtain two additional rabies immunizations if exposure to rabies occurs. The high cost of postexposure prophylaxis reinforces the importance of having adequate insurance to cover health expenses abroad, including evacuation.
Cholera and Plague Vaccines
Vaccines for plague and cholera are available in the United States, but both have low efficacy. The risk of travelers acquiring plague and cholera is low. These vaccines are rarely indicated.
Learning More About Travel Medicine
Travel medicine is emerging as a distinct specialty because of ever-changing global disease patterns and increased world travel. Travel medicine recommendations are modified frequently, so up-to-date sources should always be reviewed before specific travel advice is given to patients planning trips abroad.
Travel medicine offers the family physician a new niche in a diverse specialty. The resources listed in Table 1 provide useful information for physicians who want to develop expertise in travel medicine.