
Am Fam Physician. 1998;58(5):1163-1172
The American Geriatric Society currently recommends screening mammography for women up to 85 years of age whose life expectancy is three years or longer. The value of clinical breast examinations in older women needs further study. Total mastectomy and partial mastectomy with postoperative radiation therapy yield similar results in localized breast cancer. Postoperative irradiation may be avoided in women with small tumors (2.5 cm or less in diameter) who have undergone quadrantectomy. Lymph node dissection is important for tumor staging but significantly increases the risks and morbidity of surgery. Lymph node mapping may obviate the need for lymphadenectomy in many older women. Adjuvant hormonal therapy for at least two years appears to be beneficial in all women with hormone-receptor–rich tumors. Adjuvant chemotherapy is indicated in women with lymph node involvement or high-risk tumors with no lymph node involvement. Unless life-threatening metastases are present, hormonal therapy is the first approach to metastatic cancer. Chemotherapy is indicated if endocrine therapy is unsuccessful or life-threatening metastases are present. Most chemotherapy regimens appear to be well tolerated, even by women over 70 years of age. Special treatment should be employed for metastases to tumor sanctuaries (i.e., brain, eyes), the long bones, the spine and the chest wall.
Breast cancer is the most common cause of cancer deaths in women over 65 years of age.1 The multidisciplinary approach to this cancer includes prevention, early detection through appropriate screening, treatment of localized tumors and management of advanced disease. The family physician may assume a key role in these interventions by providing education and support to patients and their families. The removal of age-related barriers to breast cancer prevention and treatment is also an important task of the family physician.1,2
Epidemiology
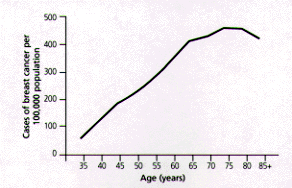
More than 50 percent of breast cancers occur in women 65 years of age and older.1 With increasing age, the risk of comorbid conditions and cancer-related death also increases.5,6 As many as 50 percent of women 65 years of age and older who develop breast cancer die of the disease.1 Even when a woman survives, breast cancer causes significant morbidity and disability.
Decisions related to the prevention and treatment of breast cancer in older women need to be individualized.4 Factors that must be considered include the woman's risk of breast cancer, the aggressiveness of the cancer and the woman's life expectancy.
Risk Factors for Breast Cancer in Older Women
Known and possible risk factors for breast cancer in postmenopausal women are listed in Table 1. Family history appears to be a risk factor even for older women.7 However, familial prevalence does not necessarily imply inheritance, because environmental conditions in the same household (e.g., dietary habits, substances in the air) hypothetically may cause a cluster of cancers in a family. Only a negligible proportion of breast cancers in older women are associated with known genetic mutations.
| Age | |
| Family history | |
| Endocrine history | |
| Early menarche and late menopause | |
| Late age of first completed pregnancy | |
| Number of pregnancies | |
| Android obesity | |
| Hormone replacement therapy | |
| Alcohol use | |
| Nutritional factors (e.g., dietary fat) | |
| Ionizing radiation | |
Endocrine risk factors for breast cancer are dominated by unopposed exposure to estrogen. These risks include early menarche, late menopause, late first pregnancy, android obesity and hormone replacement therapy.1 Android obesity, defined as a ratio of 0.71 or greater between the body circumferences measured at the waist and the hips, is associated with decreased circulating concentrations of sex-hormone–binding globulin and an increased concentration of free estrogen.8
A particular concern in older women is hormone replacement therapy, which currently involves the administration of estrogen alone or in combination with progestin in an effort to minimize the risk of endometrial cancer.9 The role of hormone replacement therapy in the pathogenesis of breast cancer is controversial. Some studies10–12 indicate that the incidence of breast cancer increases somewhat with the estrogen dosage and the duration of treatment. Other studies suggest that the increased incidence of breast cancer observed in women who have received long-term hormone replacement therapy may be related to the more prolonged survival of these women.12 Conjugated estrogens in dosages of 0.625 mg or less are not associated with breast cancer,11,12 but these dosages may be too low to ameliorate the symptoms of menopause, including hot flushes and vaginal atrophy. No evidence exists that the addition of progestin influences the risk of breast cancer.
The risks and benefits of hormone replacement therapy should be carefully balanced. One detailed analysis9 demonstrated that hormone replacement therapy has positive effects on survival and quality of life in virtually all circumstances. Hormone replacement is certainly indicated in women with a family history of coronary artery disease and/or severe menopausal symptoms. While alternative approaches to osteoporosis prevention are now available, estrogen replacement still seems to represent the best tested therapy for managing this condition.
Alcohol consumption has a definite etiologic role in breast cancer.7 The association between dietary fat and breast cancer is controversial. Finally, exposure to ionizing radiation increases the risk of breast cancer.
Pathophysiology in Older Women
According to both clinical observations5 and laboratory studies,13 breast cancer becomes more indolent in the aged. Thus, as women age, the prevalence of non–life-threatening metastases appears to increase, while the incidence of life-threatening metastases tends to decrease. The risk of death due to metastatic disease also decreases with advancing age.5
One explanation for the more indolent course of breast cancer in older women is that the prevalence of hormone-receptor–rich, well-differentiated and slowly proliferating neoplasms increases with age. The ability of the host to support tumor growth may also decrease with age.13
Prevention and Early Detection
Chemoprevention with estrogen antagonists is the most promising form of primary breast cancer prevention in older women. A recent meta-analysis14 reviewing the effects of adjuvant tamoxifen therapy in approximately 75,000 women with early breast cancer showed a 36 percent reduction in new contralateral cancers in the tamoxifen-treated group over a 15-year follow-up period. Three ongoing clinical trials in the United States, the United Kingdom and Italy are examining the potential benefits of tamoxifen for breast cancer prevention in postmenopausal women.15 However, the risks of prolonged tamoxifen administration, including deep venous thrombosis, endometrial cancer, hot flushes and vaginal discharge, may limit the use of this preventive strategy.
Secondary prevention through the screening of asymptomatic women is the most established form of breast cancer prevention. Aging may affect screening results. Since the prevalence of breast cancer increases with age, the positive predictive value of screening tests may improve with the age of the population screened. Conversely, the death-preventing effects of screening may progressively wane due to competing causes of death. The benefits of screening mammography are first seen three to seven years after the initial mammography.16 Thus, women with a life expectancy of three years or longer may theoretically benefit from screening.
One area that merits further study is the effect of breast cancer screening on quality of life in older women. Early diagnosis of breast cancer may prevent the complications of locally advanced disease.
Currently available screening tests include breast self-examination, clinical examination of the breast and mammography. The value of breast self-examination in reducing breast cancer mortality is marginal.16 At best, self-examination may heighten the awareness of breast cancer and promote health maintenance. Physicians and patients should be well aware of the low sensitivity of breast self-examination and the fact that it cannot substitute for more effective screening strategies.
When performed by a trained health care professional, the clinical breast examination may detect some cancers not found by mammography.17 The clinical breast examination requires no special equipment and may be performed during any office visit. The examination appears to be particularly convenient with older women, who tend to see their physician on a regular basis.
In both the Breast Cancer Detection Demonstration Project and the Canadian study, most tumors diagnosable by mammography only were carcinomas in situ with negligible metastatic potential.17 Furthermore, the addition of mammography to clinical examination of the breast in the Canadian study failed to reduce the mortality rate for breast cancer in women 50 to 59 years of age. The positive predictive value of the clinical breast examination may improve with the age of the patient, due to the increased prevalence of breast cancer and the development of more atrophic breasts.
At least eight prospective controlled trials and five historically controlled trials have examined the value of serial screening mammography in asymptomatic women.18 In the majority of these trials, screening mammography led to a 20 to 30 percent reduction in the breast cancer mortality rate for women 50 to 65 years of age. The benefits of mammography in women older than 65 years have been less well established, primarily because this age group was excluded or underrepresented in most of the trials.
The American Geriatric Society currently recommends that screening mammography be performed in older women up to 85 years of age if their life expectancy is three years or longer. This approach has been supported by a recent decision analysis.19
Social and cultural barriers once limited the use of cancer screening in most older women. In the past decade, however, some form of breast cancer screening has been performed in the majority of older women, in part because of public education efforts. Support from family physicians appears to be essential to removing any remaining barriers to screening.2
General Principles of Treatment
Breast cancer may be treated with surgery,irradiation, hormonal agents and chemocytotoxic drugs. These modalities may be used separately or in combination, based on the stage of the disease and the characteristics of the patient.20
Surgery
Surgery includes treatment of the primary breast tumor and dissection of the ipsilateral axillary lymph nodes. Currently, primary tumors are seldom treated with radical mastectomy. A total mastectomy spares the pectoralis minor muscle, while a partial mastectomy (i.e., lumpectomy) is targeted at preserving the breast. Types of lumpectomy include quadrantectomy and lesser procedures. Both total mastectomy and partial mastectomy may be performed under local anesthesia.20
Lymph node mapping may eventually make lymph node dissection obsolete.21 This technique allows intraoperative identification and sampling of the so-called “sentinel lymph node” (the first lymph node receiving lymphatic drainage from the breast). When the sentinel lymph node has no tumor involvement, the risk of other lymph node involvement appears to be negligible.22 More studies are needed to confirm these findings.
Radiation Therapy
Radiation therapy for breast cancer involves external-beam irradiation. It is generally used in addition to surgery or for the palliation of pain.
Hormonal Therapy
Hormonal therapy may be used as an adjuvant treatment for breast cancer. It is also used to prevent systemic recurrence of localized disease and to manage metastatic cancer. In general, hormonal therapy is not indicated for treatment of tumors that are poor in hormone receptors. The dosages and side effects of the hormonal agents most commonly used to treat breast cancer are listed in Table 2.
| Drug class | Drug | Dosages | Complications |
|---|---|---|---|
| Estrogen antagonists | Tamoxifen (Nolvadex) | 20 mg per day | Hot flushes, drying of vaginal secretions, deep venous thrombosis, retinopathy, hypercalcemia* |
| Progestins | Megestrol (Megace) | 40 mg four times daily | Weight gain, nausea, fluid retention |
| Aromatase inhibitors | Aminoglutethimide (Cytadren)† | 250 mg twice daily | Somnolence, skin rash, hypoadrenalism |
| Anastrozole (Arimidex) | 1 mg per day | None | |
| Letrozole (Femara) | 50 mg (one tablet) per day | None | |
| Estrogens | Diethylstilbestrol | 5 mg three times daily | Deep venous thrombosis, fluid retention, hypercalcemia* |
| Androgens | Fluoxymesterone (Halotestin) | 10 mg three times daily | Virilization, fluid retention |
The long-term effects of tamoxifen are of special interest because the drug has been used extensively in the adjuvant management of breast cancer.23 Tamoxifen has some estrogen-like activities that may be particularly beneficial in older women. These include delay of osteoporosis and prevention of coronary deaths. However, the potential association of tamoxifen and endometrial cancer has raised some serious concerns about long-term therapy.24 Tamoxifen may increase the risk of endometrial cancer by 20 to 40 percent.24 Endometrial cancer can usually be diagnosed at an early stage and completely resected. If the patient undergoes serial transvaginal ultrasound examinations, early detection is more likely. Mortality from tamoxifen-induced endometrial cancer has been negligible.24
Anastrozole (Arimidex) is a new aromatase inhibitor that is being used to treat breast cancer. This compound is better tolerated than aminoglutethimide (Cytadren), and corticosteroid supplementation is not required.
Cytotoxic Chemotherapy
Cytotoxic chemotherapy has a major role in the adjuvant treatment of breast cancer and the management of metastatic disease. Commonly used cytotoxic drugs include cyclophosphamide (Cytoxan), methotrexate (Rheumatrex), fluorouracil, the anthracyclines (doxorubicin [Adriamycin, Rubex] and epirubicin), mitoxantrone (Novantrone), the taxanes (paclitaxel [Taxol] and docetaxel [Taxotere]) and vinorelbine (Navelbine).20 The few studies that have explored the tolerance of breast cancer chemotherapy found a comparable incidence of complications in women both older and younger than 65 years of age.25–27 However, the older women in these studies had negligible comorbidity and therefore may not be representative of the older population at large. Furthermore, very few of the women were over 80 years of age. Thus, virtually no information is available concerning the tolerance of chemotherapy by the very aged.
Some important steps may be taken to minimize the complications of cytotoxic chemotherapy in older women. First, the dosages of renally excretable drugs, such as methotrexate, should be adjusted to the creatinine clearance in the individual patient.25 Second, when alternative drugs are available, the agent with the lowest risk of complications should be selected. For example, mitoxantrone and doxorubicin have comparable activity, but mitoxantrone appears to be better tolerated in terms of cardiac toxicity, alopecia, nausea, vomiting and fatigue.28 Third, antidotes to chemotherapy toxicity, such as hemopoietic growth factors to ameliorate myelotoxicity29 or desrazoxane (Zinecard) to prevent anthracycline cardiotoxicity,30 may make chemotherapy more tolerable in older patients.
Treatment Based on Breast Cancer Stage
Carcinoma In Situ (Stage 0)
Ductal carcinoma in situ is treated with mastectomy or partial mastectomy followed by postoperative irradiation.1 Since lymph node involvement is rare in ductal carcinoma in situ, routine lymph node dissection is not indicated. This cancer is often multicentric and is associated with a high risk of local recurrence after partial mastectomy. It is extremely important that local recurrences be controlled, because they may be associated with invasive and metastatic cancer.3 One recent study31 showed that local recurrences are more likely with large tumors (2.5 cm or more in diameter) with aneuploidy and poor histologic differentiation.
Lobular carcinoma in situ has similar risks of local and contralateral recurrence. Thus, it is managed with either local excision or bilateral mastectomy. Local excision with mammographic follow-up appears to be the most sensible treatment.1
Localized Cancer (Stages I and II)
The treatment of localized breast cancer involves control of the primary tumor, lymph node dissection and, in selected cases, systemic therapy. The same degree of local control is achieved with total mastectomy or partial mastectomy in combination with postoperative irradiation.1
In specific clinical situations, adjuvant systemic therapy prolongs both disease-free survival and overall survival in patients with localized breast cancer.14 The decision to use systemic treatment is determined by the risk of systemic recurrence (Table 3). Treatment guidelines formulated by an international consensus panel (St. Gallien conference) are presented in Table 4.33 Age may affect all aspects of localized breast cancer management. Major controversial issues include the use of postoperative radiation therapy after partial mastectomy, nonoperative management of primary breast cancer and adjuvant systemic therapy.
| With axillary lymph node involvement: | |
| Number of involved lymph nodes | |
| Increased expression of HER/neu oncogene | |
| Without axillary lymph node involvement: | |
| Tumor size | |
| Hormone receptor concentration | |
| Histologic grade | |
| Tumor cell proliferation | |
| Degree of neovascularization | |
Postoperative irradiation reduces the local recurrence rate and allows preservation of the breast, but it does not appear to affect patient survival.1 Furthermore, postoperative irradiation increases the cost of treatment. The six weeks of daily radiation treatments may be very inconvenient for older women. The local recurrence rate for tumors with a diameter of 2.5 cm or less may be only 3 percent at five years in women 55 years of age and older.34 Thus, postoperative irradiation may be unnecessary in older women with small tumors who have undergone quadrantectomy.
Of the studies that have explored the non-surgical management of localized breast cancer in women 70 years of age and older, the Cancer Research Campaign Trial35 has produced the most definitive results. In this trial, patients were randomized to receive tamoxifen therapy alone or to undergo partial mastectomy with adjuvant tamoxifen therapy. Of the patients treated nonsurgically, 40 percent had disease progression requiring surgery within two years. The nonsurgical approach appears to be inferior to standard treatment and therefore should be used only in patients in whom surgery is contraindicated.
Controversy also exists concerning indications for tamoxifen therapy and the duration of this treatment. The indications for adjuvant systemic chemotherapy in older women with localized breast cancer are also controversial. Adjuvant tamoxifen therapy has been found to prolong both disease-free survival and overall survival in all postmenopausal women with localized breast cancer,14 including those older than 70 years of age. A minimum of two years of adjuvant tamoxifen treatment is necessary to affect patient survival,14 and additional benefits may be seen with up to five years of treatment.1
The National Cancer Institute recently recommended that adjuvant tamoxifen therapy not be continued beyond five years, except in ongoing clinical trials. In part, this recommendation was based on the fact that a recent update of the National Surgical Adjuvant Breast and Bowel Project B-14 comparing five and 10 years of tamoxifen therapy failed to demonstrate any benefits from treatment beyond five years.1 Other influencing factors were the concerns about tamoxifen-induced endometrial cancer and the development of tamoxifen resistance.
Three questions summarize the controversies related to adjuvant chemotherapy: Is chemotherapy of any value in older women? Which form of chemotherapy, if any, should be used? Which conditions are indications for adjuvant chemotherapy?
Several controlled clinical trials have shown that adjuvant chemotherapy delays breast cancer recurrence in postmenopausal women, but none of the trials has conclusively demonstrated a reduction in the breast cancer mortality rate.1 A meta-analysis14 of several trials showed a 13 percent decrease in the breast cancer mortality rate for women 50 to 59 years of age and a 10 percent reduction for women 60 to 69 years of age. No reduction in mortality occurred in women 70 years of age and older, although this age group represented a small minority of the study subjects.
The age-related decline in mortality reduction is not surprising, considering the increased prevalence of other causes of death. Thus, survival alone may not be an appropriate parameter to test the value of adjuvant chemotherapy in older patients. Measures incorporating quality of life may be more appropriate.
With respect to determining which chemotherapeutic agents are more effective, combinations that include doxorubicin appear to be preferable in older women with positive axillary lymph nodes. In women with negative lymph nodes, less toxic combinations, such as methotrexate and fluorouracil, may be adequate.1
The St. Gallien conference provided an adequate set of indications for adjuvant chemotherapy in older women with breast cancer.33
Locally Advanced Cancer (Stage III)
Older women seldom present with locally advanced disease. In most cases, stage IIIA disease results from the neglect of a smaller tumor. The breast cancer is then managed with surgery and adjuvant chemotherapy. Surgical treatment, including mastectomy with postoperative irradiation of the chest wall, may be required to prevent chest wall recurrence.
Since stage IIIB breast cancer is very aggressive, local treatment alone is of limited benefit. Current treatment of stage IIIB breast cancer includes preoperative chemotherapy (and hormonal therapy, if the tumor is rich in hormone receptors), followed by surgery and radiation therapy. Chemotherapy should include an anthracycline. With this approach, a five-year survival rate of 30 to 40 percent may be obtainable.1
Metastatic Cancer (Stage IV)
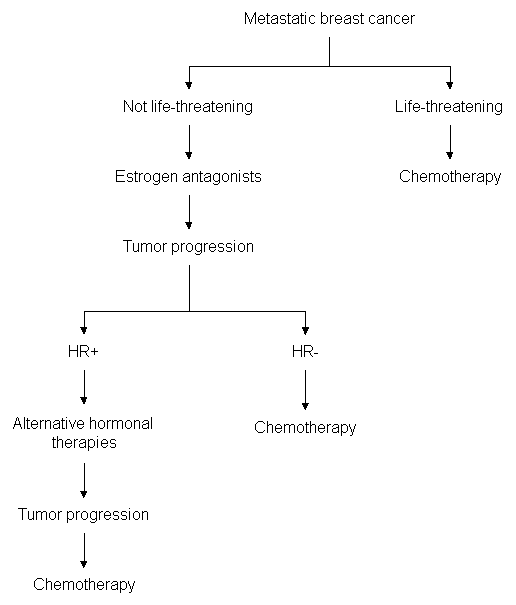
Metastatic breast cancer requires systemic treatment. Estrogen antagonists are front-line agents if the patient has not been exposed to them previously and if life-threatening metastases (hepatic or lymphangitic lung metastases) are not present.1 If the tumor progresses during treatment with estrogen antagonists, alternative hormonal therapies may be used to treat hormone-receptor–positive tumors, and chemotherapy may be used to treat hormone-receptor–poor tumors (Figure 2). In the presence of life-threatening metastases, chemotherapy is generally indicated, because this treatment works more rapidly than hormonal therapy.
Metastases to tumor sanctuaries, such as the brain or the eyes, require radiation therapy. A single brain metastasis may also be resected.
Metastases to the long bones increase the risk of pathologic fractures, while metastases to the spine may cause spinal cord compression. When 50 percent or more of the cortex of a long bone is destroyed, orthopedic fixation of the bone is indicated.36 While waiting for surgery, the patient should be instructed not to bear weight on the at-risk bone. The use of crutches or a wheelchair is recommended. Painful metastases to the spine should be evaluated with myelography or magnetic resonance imaging. In the presence of epidural extension, emergency radiation therapy and corticosteroid treatment are indicated.
Single chest-wall metastasis requires both local and systemic treatment.1 Local treatment involves surgery (when feasible) or radiation therapy. The duration of systemic treatment has not been definitely established. Hormonal therapy may be used for at least two years to treat hormone-receptor–rich tumors. Six courses of chemotherapy may be used to treat hormone-receptor–poor tumors. With this approach, more than 50 percent of patients are alive and free of disease five years from the time of chest-wall recurrence.