
A more recent article on glaucoma is available.
Am Fam Physician. 1999;59(7):1871-1879
See related patient information handout on using glaucoma eyedrops, written by the authors of this article.
Medication classes historically used in the management of glaucoma include beta blockers, miotics, sympathomimetics and carbonic anhydrase inhibitors. Because topically applied medications are more site specific, they are preferred in the treatment of glaucoma. Compared with oral medications, topical agents are associated with a decreased incidence of systemic side effects. With topical administration, conjunctival and localized skin allergic reactions are relatively common, whereas severe reactions, including death, are rare. Recently introduced topical agents for glaucoma therapy include dorzolamide and brinzolamide, the first topical carbonic anhydrase inhibitors; brimonidine and apraclonidine, more ocular-specific alpha agonists; and latanoprost, a prostaglandin analog, which is a new class of glaucoma medication. Latanoprost has the unique side effect of increasing iris pigmentation. Like their predecessors, the newer agents lower intraocular pressure by a statistically significant degree. Preservation of visual field, the more substantial patient-oriented end point, continues to be studied.
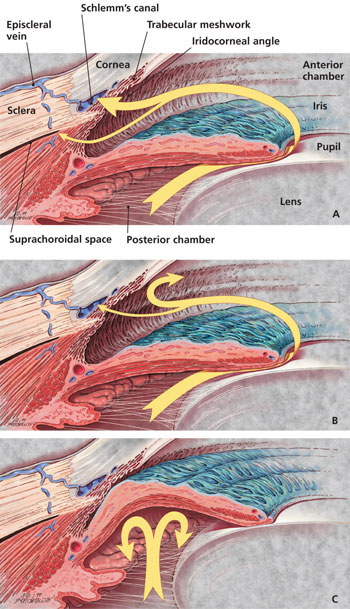
Based on the configuration of the anterior chamber angle of the eye (Figure 1a), glaucoma is classified as open angle or closed angle (Figures 1b and 1c), with a number of possible subdivisions.1 This article reviews topical therapies for primary open-angle glaucoma, the most common form of the disease. Detailed reviews of the classification and pathogenesis of glaucoma are available elsewhere.1–5
More than 2 million Americans have glaucoma.3 This ocular disease is frequently asymptomatic at the time of diagnosis, but it can result in progressive visual field loss and eventual blindness. Primary open-angle glaucoma is generally diagnosed in persons over the age of 40 years and is five times more common in blacks than in whites.6 This condition is the second leading cause of irreversible blindness in the United States and the leading cause of loss of vision in African Americans.3
Efforts to prevent visual field loss caused by glaucoma have been hampered by the limitations of screening techniques7 and uncertainties about the pathogenesis and treatment of the disease.8–13 Based on our present understanding of the optic nerve damage that leads to visual field loss characteristic of glaucoma, increased intraocular pressure (IOP) is the sole risk factor amenable to current medical and surgical treatments. Several trials sponsored by the National Institutes of Health are currently investigating the ability of glaucoma medications to preserve visual function and are assessing the role of surgical intervention as initial or subsequent therapy.14
Glaucoma and IOP elevation are often erroneously considered synonymous. In actuality, glaucoma is characterized by damage to the optic nerve that results in visual field loss regardless of the IOP level at which the damage occurs. IOP is within the statistically “normal” range (less than 21 mm Hg) in more than one of six patients with glaucoma.3 This condition is termed “normal-tension glaucoma.” Conversely, many patients with elevated IOP show no evidence of optic nerve damage or visual field loss. This condition is known as “ocular hypertension.” Much debate exists as to when glaucoma therapy should be initiated in patients with mild ocular hypertension alone.
General Principles of Glaucoma Therapy
When glaucoma is diagnosed, whether in the presence of normal or elevated IOP, it is treated. In the United States, medications are typically first-line therapy. Elsewhere in the world, notably in Great Britain, initial treatment with surgery is becoming increasingly common.15 The agents currently used to treat glaucoma are designed to decrease IOP. These drugs limit aqueous humor production in the ciliary body and/or enhance aqueous outflow through the trabecular meshwork or uveoscleral pathways (Figures 1a, 1b and 1c).
When a topical medication is chosen as first-line therapy in a patient with primary open-angle glaucoma, a stepped approach is used. Thus, a single topical agent is given up to its maximum dosage, as tolerated, before another agent is added or a different agent is tried. Additional medications are selected based on their potential complementary contribution to IOP reduction and the tolerability of their side effects. The patient who is receiving maximal medical therapy may be applying up to four different classes of topical medications. Several combination drops have been marketed or are undergoing trials that will simplify dosage regimens and thereby increase patient compliance.
Family physicians should be familiar with the primary pathway for the systemic absorption of topical glaucoma medications (Figure 2) and the potential for side effects. Drugs administered to the eye pass rapidly through the nasolacrimal duct into the nose and then through the highly vascular nasal mucosa. Thus, they enter the systemic circulation without benefit of first-pass metabolism in the liver.
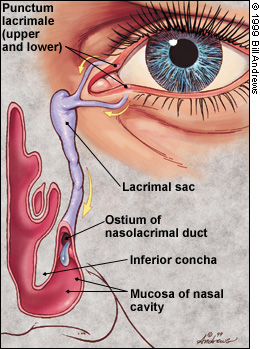
The systemic absorption of topically applied medications may result in adverse effects, even death in rare instances. Systemic side effects are more likely to occur in patients who are taking other medications, notably antihypertensive and antiarrhythmic drugs.
Both patients and physicians may neglect to list topical antiglaucoma agents as medications. Thus, family physicians must ask patients about ocular medications when a medication history is requested and keep medication records current. Documentation of ocular medication use can be valuable in the evaluation of such common presentations as conjunctivitis, asthma, syncope, depression and sexual dysfunction.
Medications for Topical Administration
BETA BLOCKERS
Topically administered beta blockers have been the mainstay of glaucoma therapy for more than two decades. Timolol maleate (Timoptic) is the standard agent against which other medications are measured in terms of efficacy, side effects and cost.16 Beta blockers are thought to lower IOP mainly by decreasing aqueous humor production in the ciliary body of the eye. They may also induce a slight increase in aqueous outflow.
Although topically administered timolol is frequently recommended as first-line therapy, the actions and side effects of this drug may limit its use. Timolol and other topically applied beta blockers have been associated with asthma exacerbation, including status asthmaticus, worsening congestive heart failure, heart block and, rarely, sudden death17 (Table 1). Because these agents may block the typical systemic manifestations of hypoglycemia, they should be used with caution in patients with diabetes mellitus.
| Topical medications | Ocular side effects | Systemic side effects | Contraindications | Drug interactions | Clinical notes | Cost for one container* | |
|---|---|---|---|---|---|---|---|
| Beta blockers | |||||||
| Nonselective | |||||||
| Timolol maleate (Timoptic, Timoptic-XE) | Burning/stinging; transient blurred vision (increased with gel); photophobia; conjunctivitis; blepharitis; punctate keratitis; contact dermatitis; eyelid erythema | Decreased heart rate/cardiac output; bronchospasm; hypotension; depression; decreased libido; impotence; worsened lipid profile; decreased stress response to hypoglycemia, surgery or anaphylaxis | Asthma; chronic obstructive pulmonary disease; congestive heart failure; sinus bradycardia; second- or third-degree atrioventricular block; hypersensitivity to beta blockers | Use cautiously with oral beta blockers, calcium channel blockers, quinidine, digitalis and catecholamine-depleting drugs (e.g., reserpine) | Lack of nocturnal effect; tachyphylaxis | Timoptic: 0.25 %, 5 mL, $16† Timoptic-XE: 0.25 %, 5 mL, $23 | |
| Levobunolol (Betagan) | Increased relative to timolol | Same as timolol | Same as timolol, plus hypersensitivity to sulfite preservative | Same as timolol | Largest drop size | 0.25 %, 5 mL, $17† | |
| Carteolol (Ocupress) | Same as timolol | Same as timolol | Same as timolol | Same as timolol | Some intrinsic sympathomimetic activity | 5 mL, $19 | |
| Metipranolol (Optipranolol) | Has the most ocular side effects of the beta blockers in this class; anterior uveitis | Same as timolol | Same as timolol | Same as timolol | 5 mL, $13 | ||
| Selective | |||||||
| Betaxolol (Betoptic, Betoptic S) | Increased relative to timolol | Rare; fewer cardiopulmonary side effects than timolol | Sinus bradycardia; second- or third-degree atrioventricular block; overt congestive heart failure | Same as timolol, plus may antagonize adrenergic psychotropic drugs such as thioridazine (Mellaril) | Betoptic: 5 mL, $21 Betoptic S: 5 mL, $21 | ||
| Miotics | |||||||
| Pilocarpine (Isopto Carpine, Ocusert Pilo) | Burning; blurred vision; difficulty with night vision; miosis or accommodative spasm; lens opacity (rare); retinal detachment (rare); precipitation of closed-angle glaucoma (rare) | Sweating; salivation; urinary frequency; nausea; diarrhea; bronchospasm; biliary colic; mental status change; variable cardiovascular response | Hypersensitivity; poorly controlled asthma; acute iritis | May precipitate if administered with sodium sulfacetamide | Blurred vision increased with gel; miosis decreased with Ocusert Pilo; retinal detachment increased in patients with myopia; use with caution in patients with cataracts, hyperthyroidism, parkinsonism or urinary tract obstruction | Isopto Carpine: 0.25 %, 15 mL, $14† Ocusert Pilo: $40‡ | |
| Carbonic anhydrase inhibitors | |||||||
| Dorzolamide (Trusopt) | Burning; punctate keratitis; ocular allergies; increased ocular side effects relative to timolol | Bitter taste; headache; nausea; asthenia; kidney stones (rare) | Hypersensitivity to sulfonamides; eye injury or surgery | Not recommended for use with systemic carbonic anhydrase inhibitors; may accentuate side effects of salicylate therapy; may promote excretion of acidic drugs and inhibit renal excretion of basic drugs | Contact lenses must be removed; may be reinserted 15 minutes after installation of medication; not studied in patients in patients with hepatic or renal dysfunction | 2 %, 5 mL, $22 | |
| Brinzolamide (Azopt) | Possibly decreased ocular side effects compared with dorzolamide; blepharitis; foreign-body sensation | Bitter taste; headache; rhinitis sensation | Hypersensitivity to sulfonamides | Same as dorzolamide | Same as dorzolamide | 1 %, 5 mL, $20 | |
| Sympathomimetics | |||||||
| Epinephrine-like Dipivefrin (Propine) | Burning; follicular conjunctivitis; macular edema in patients who are aphakic | Increased blood pressure; arrhythmias; tremor | Narrow-angle glaucoma | Use cautiously with cardiovascular or stimulant medications | Epinephrine prodrug; decreased ocular and systemic side effects relative to epinephrine; use cautiously in patients with cardiovascular disease | 0.1 %, 5 mL, $17† | |
| Clonidine-like | |||||||
| Brimonidine (Alphagan) | Conjunctival blanching; ocular allergy (less than with apraclonidine, more than with timolol) | Headache; drowsiness; fatigue; variable blood pressure response | Hypertensive crisis; therapy with monoamine oxidase inhibitors; hypersensitivity to clonidine (Catapres) | Use cautiously with antihypertensive medications and digitalis | Tachyphylaxis (less common); not studied in patients with hepatic or renal dysfunction | 0.2 %, 5 mL, $22 | |
| Apraclonidine (Iopidine) | Allergic/local reaction; transient change in visual activity | Increased central nervous system effects change visual activity | Same as brimonidine | Same as brimonidine | Tachyphylaxis (common); short-term adjunctive agent, not first-line therapy | 0.5 %, 5 mL, $12 | |
| Prostaglandin analogs | |||||||
| Latanoprost (Xalatan) | Burning/stinging; iris pigmentation; punctate keratitis | Headache; symptoms of upper respiratory infection; chest pain (rare); myalgias (rare) | Hypersensitivity; narrow-angle closure; ocular infection or inflammation | May be precipitated by eyedrops that contain thimerosol (antiseptic) | Prodrug; diurnal decrease in intraocular pressure; not studied in patients with hepatic or renal dysfunction | 0.005 %, 2.5 mL, $42 | |
Central beta blockade from ocular application of these agents may also result in dysthymia or frank depression, as commonly occurs with orally administered beta blockers. Impotence is another well recognized side effect of topically applied beta blockers. This adverse effect may cause patients to stop using these medications. Such patients may be reluctant to discuss this discontinuation or the reason behind it with their physician.
Betaxolol (Betoptic), a cardioselective beta blocker, has a more favorable cardiopulmonary side effect profile than timolol. Because timolol has a superior IOP-lowering effect, it is frequently recommended over betaxolol when cardiopulmonary compromise is not of concern. Nevertheless, several studies18–20 demonstrate that betaxolol provides superior visual field preservation. Betaxolol is marketed in a suspension (Betoptic S) with a lower medication concentration and a reportedly decreased incidence of systemic side effects compared with the corresponding solution.
Other topically applied beta blockers include metipranolol (Optipranolol), carteolol (Ocupress) and levobunolol (Betagan). The manufacturer of carteolol claims that the drug has an intrinsic sympathomimetic effect; therefore, it theoretically may have fewer cardiopulmonary side effects than timolol. At present, however, physicians should not rely on this potentially lessened risk in selecting a topical medication for glaucoma treatment.
Beta blockers are typically applied twice daily, although once-daily therapy may be effective in some patients. A recently introduced gel-forming solution of timolol maleate (Timoptic-XE) has the advantage of once-daily dosing. This solution is likely to become the therapy of choice in patients who can tolerate beta blockers.
MIOTICS
Pilocarpine (Isopto Carpine), the principal miotic used in glaucoma therapy, was isolated from the leaves of Pilocarpus plants in the 19th century. It was first used for glaucoma therapy in 1956. Miotics (acetylcholine agonists and cholinesterase inhibitors) are thought to promote increased trabecular aqueous outflow by contracting the ciliary muscle of the eye.
Side effects such as accommodative spasm, brow-ache and myopia are more pronounced in younger patients who are treated with miotics. In patients with cataracts, miotics may contribute to functional disability by decreasing daytime and, perhaps more significantly, nighttime vision. Systemic cholinergic effects such as nausea, vomiting, sweating and cutaneous vasodilatation may occur.
Pilocarpine therapy is relatively inexpensive. Nevertheless, the high incidence of ocular side effects and the inconvenience of dosing four times daily make pilocarpine less popular than other agents used in the medical management of glaucoma.21 Pilocarpine in a continuous-release vehicle (Ocusert Pilo) applied once weekly to the lower conjunctival sac is promising in theory but has not gained popularity, in part because it tends to fall out of the eye.
CARBONIC ANHYDRASE INHIBITORS
Orally administered carbonic anhydrase inhibitors have long been used in the management of primary open-angle glaucoma refractory to other forms of medical therapy. Agents such as acetazolamide (Diamox) and methazolamide (Neptazane) decrease aqueous humor secretion by the ciliary epithelium.
The use of carbonic anhydrase inhibitors is limited by side effects ranging from general malaise to symptomatic metabolic acidosis, renal calculi and bone marrow suppression. Orally administered carbonic anhydrase inhibitors may accentuate the effects of diuretics and contribute to volume depletion and clinically significant hypokalemia. Concomitant use with aspirin increases the risk of salicylate toxicity.
Dorzolamide (Trusopt) and brinzolamide (Azopt) are the first topical carbonic anhydrase inhibitors labeled by the U.S. Food and Drug Administration (FDA) for the treatment of primary open-angle glaucoma. Each drug is used two to three times daily. Dorzolamide is also marketed in combination with timolol (Cosopt). These agents are favored over oral agents because of their greater site specificity and markedly fewer systemic side effects.
Like acetazolamide, dorzolamide and brinzolamide are sulfonamide derivatives. As such, the potential exists for bone marrow dyscrasias, transaminitis and dermatologic reactions ranging from simple hypersensitivity to Stevens-Johnson syndrome. To date, however, the topical agents have not been associated with these adverse effects. Dorzolamide and brinzolamide should not be used in patients with a history of hypersensitivity to sulfa medications, and their use is not recommended in patients with moderate to severe renal impairment.
Established systemic side effects associated with topically administrated dorzolamide and brinzolamide include bitter taste (experienced by up to 25 percent of patients),22 headache, nausea, asthenia and fatigue. Rarely, nephrolithiasis may occur. The manufacturer claims a lower incidence of ocular side effects for brinzolamide.23 However, added experience with brinzolamide is required to determine if this stated advantage is supported by repeated clinical use.
SYMPATHOMIMETICS
Topical sympathomimetics may be divided into epinephrine (alpha- and beta-receptor stimulation) and clonidine-like agents (alpha-receptor stimulation). Sympathomimetics either decrease aqueous production or increase aqueous outflow. Epinephrine has frequent ocular allergic side effects and consequently is less commonly used in patients with glaucoma. Dipivefrin (Propine), an epinephrine prodrug, is taken twice daily. Although dipivefrin produces fewer ocular and systemic side effects than epinephrine, it is being supplanted by clonidine-like agents for glaucoma therapy.
The FDA has labeled apraclonidine (Iopidine) for use in the management of transient IOP elevations after ocular surgery. This agent is associated with a high incidence of tachyphylaxis (loss of effect) and clonidine-like central nervous system (CNS) effects such as somnolence and orthostasis. Ocular allergic side effects are common. Thus, apraclonidine has only limited use in the chronic management of primary open-angle glaucoma.
Brimonidine (Alphagan) is approved for maintenance glaucoma treatment and may be suitable as monotherapy.24 This drug has fewer CNS and ocular side effects than apraclonidine. The increased selectivity of brimonidine for alpha2-receptor sites is postulated to decrease IOP by limiting aqueous production and facilitating increased outflow via the uveoscleral pathway. Tachyphylaxis occurs less frequently with brimonidine than with apraclonidine. Potential limitations to the use of brimonidine include its dosing schedule (two or three times daily) and its cost. Coadministration with monoamine oxidase inhibitors is contraindicated because of the risk of precipitating a hypertensive crisis.
PROSTAGLANDIN ANALOGS
Latanoprost (Xalatan) was recently labeled for use in patients with glaucoma. This agent is one of the prostaglandin analogs, a new class of agents for the treatment of glaucoma. Latanoprost is taken once daily at bedtime. IOP reduction is equivalent to that achieved with twice-daily timolol therapy. Compared with timolol, latanoprost has a more favorable local and systemic side effect profile.
The development of a prostaglandin suitable for clinical use in the treatment of glaucoma was previously hampered by the prevalence of ocular side effects, primarily conjunctival hyperemia. Like dipivefrin, latanoprost (a prostaglandin F2α analog) is a prodrug that produces the desired clinical effect with a more tolerable degree of ocular side effects.
Latanoprost lowers IOP by increasing uveoscleral outflow (the minor pathway for the removal of aqueous humor from the anterior chamber of the eye). Interestingly, latanoprost reduces IOP to a greater extent when it is administered once daily in the evening than when it is applied either once daily in the morning or twice daily. Unlike timolol, latanoprost exhibits a sustained IOP-lowering effect throughout the day and night.25
Increased iris pigmentation occurs in up to one in six patients treated with latanoprost and is the main focus of discussions regarding the side effect profile of this drug.26 Patients with mixed-color irises (i.e., brown-gray or brown-green) are most at risk for this side effect, which is the result of increased melanin production. Because melanocyte division is not stimulated, the color change is not believed to place the patient at added risk for melanoma. The color change is stable and may not be reversible with discontinuation of the drug. Long-term effects are unknown.
Lash growth, another documented ocular side effect of latanoprost, is thought to be of only cosmetic significance. In case reports,27 latanoprost has also been associated with iritis, hypotony with choroidal effusion, and cystoid macular edema.
Additional potential limitations to the use of latanoprost include its cost and its container. It may be difficult for geriatric patients to use the drug's 2.5-mL dispenser bottle.
Compliance Factors
Compliance with topical glaucoma treatments depends on how effective patients perceive the therapy to be, and the number and extent of medication side effects. Comorbid ocular and systemic conditions are also likely to affect compliance. Other factors include the number of medications that have to be placed in the eye(s), the frequency of dosing, and the cost and availability of the medications. Cost comparisons are hampered by differences between measured and labeled bottle volumes and discrepancies in drop volumes for the various products.28,29 Another factor that influences efficacy is the degree to which a topically administered drug is likely to be lost because of overflow and blinking.
Future Directions
If the side effect profile of latanoprost remains tolerable—particularly if no harmful sequelae of increased iris pigmentation are found—additional prostaglandin analogs are expected to be introduced. More combination products will also be marketed for clinical use.
Nitrates and calcium channel blockers are undergoing evaluation for the management of primary open-angle glaucoma. Interest in these vasodilators stems from the hypothesis that impaired ocular blood flow contributes to the optic nerve damage that leads to glaucoma.
A possible genetic basis for treatment is also being investigated. Researchers are studying the role that a trabecular meshwork protein (identified as the TIGR protein) may play in the pathogenesis of primary open-angle glaucoma.30
Final Comments
Dorzolamide, brinzolamide, brimonidine and latanoprost are newer topical agents that can reduce IOP in patients with ocular hypertension or primary open-angle glaucoma. All four agents possess a more favorable side effect profile than their predecessors. What remains to be established through current studies is the impact these medications have on the natural history of ocular hypertension and primary open-angle glaucoma, their capacity to influence the patient-oriented end point of visual field preservation and their place in therapy relative to surgery.
Because topically applied medications are the mainstay of glaucoma therapy, patients and family physicians need to be familiar with their stated purpose and their place in glaucoma therapy, as well as their possible ocular and systemic side effects. Patients are likely to underreport their use of glaucoma medications to family physicians, and both patients and physicians may underestimate systemic adverse events that may be attributable to ocular medications.
Family physicians who are knowledgeable about the topical glaucoma medications their patients are taking and familiar with the side effects of these medications will be better prepared to anticipate and recognize the potential of these drugs to cause drug interactions and exacerbate comorbid medical conditions. Thus, if patients develop a new systemic condition or adverse effects of glaucoma medications are suspected, family physicians will be better equipped to communicate with ophthalmologists and other eye care professionals who share treatment responsibilities for these patients.