
Am Fam Physician. 1999;59(7):1885-1892
See related patient information handout on multiple myeloma, written by the authors of this article.
Multiple myeloma is the malignant proliferation of plasma cells involving more than 10 percent of the bone marrow. The multiple myeloma cell produces monoclonal immunoglobulins that may be identified on serum or urine protein electrophoresis. Bone pain related to multiple lytic lesions is the most common clinical presentation. However, up to 30 percent of patients are diagnosed incidentally while being evaluated for unrelated problems, and one third of patients are diagnosed after a pathologic fracture, commonly of the axial skeleton. Multiple myeloma must be differentiated from other causes of monoclonal gammopathy, including monoclonal gammopathy of undetermined significance, heavy chain disease, plasmacytoma and Waldenstrom macroglobulinemia. Chemotherapy with melphalan-prednisone is the standard treatment for multiple myeloma. Other treatment modalities include polychemotherapy and bone marrow transplantation. Only 50 to 60 percent of patients respond to therapy. The aggregate median survival for all stages of multiple myeloma is three years.
Multiple myeloma is a malignant proliferation of plasma cells that involves more than 10 percent of the bone marrow. It is a prototype primary malignancy of the bone associated with malignant plasma cells that secrete monoclonal immunoglobulins into the serum, the urine or both.
The family physician is often the first to identify multiple myeloma. The expertise of an oncologist is then enrolled. Other specialists join the team as needed to address specific concerns or complications. As with other malignancies, the family physician assists the patient and the family in understanding the disease and the treatment-related side effects, and in improving the patient's overall quality of life.
In 1850, MacIntyre published the first complete clinical and pathologic narration of “a case of mollities and fragilitas ossium accompanied with urine strongly charged with animal matter.” The term “multiple myeloma” was first used to describe the presence of multiple tumors originating in the bone.1
Epidemiology
Multiple myeloma is the most common primary cancer of the bones in adults. The annual incidence in the United States is three to four cases per 100,000 population. Multiple myeloma represents 1 percent of all cancers diagnosed in the United States and 10 percent of all hematologic malignancies. The median age at diagnosis of multiple myeloma is 62 years. Only 2 to 3 percent of cases are reported in patients younger than 30 years.2
Blacks in the United States are twice as likely to suffer from multiple myeloma as whites. In fact, multiple myeloma is the most common hematologic malignancy in the U.S. black population.3 Multiple myeloma is rare among persons of Asian descent, with an incidence of only one to two cases per 100,000 population.4
The risk of developing multiple myeloma appears to be higher in populations of lower socioeconomic status, particularly where diagnostic services are unavailable.5 Employment in the nuclear industry, sheet metal and agricultural occupations and jobs in which workers are exposed to wood dust increase the risk of developing multiple myeloma.6
Etiology
The exact etiology of multiple myeloma remains unclear. Occasionally it is possible to identify members of the same family with multiple myeloma, although no evidence suggests a hereditary basis for the disease.
Suggestions of an association between multiple myeloma and exposure to radiation are controversial. No evidence of an excess risk for multiple myeloma was observed among the Life Span Study cohort of atomic bomb survivors during an additional 12-year follow-up analysis using the Leukemia Registry and the Hiroshima and Nagasaki tumor registries.7 However, the incidence of multiple myeloma is higher among nuclear industry workers than in the general population.8
Farmers and horticulturists directly exposed to the pesticide DDT may have an increased risk of developing multiple myeloma.9 This substance tends to accumulate in the ecosystem and has various toxic effects in many vertebrates. Farmers are exposed to multiple herbicides and fertilizers that could increase their risk. Previous reports linking exposure to benzene with the development of multiple myeloma have been refuted.10 Preliminary research has implicated herpesvirus type 8 in the etiology of multiple myeloma.11
Pathophysiology
A precursor cell arises in patients with multiple myeloma when one or more exogenous stimuli induce cytogenetic changes in the B-cell lineage at the lymph node.12 On average, 50 percent of patients with multiple myeloma have an abnormal karyotype. Commonly detected abnormalities involve single or combinations of hyperdiploidy of chromosomes 3, 5, 7, 9, 11, 15 and 19, and hypodiploidy of chromosomes 8, 13, 14 and sex chromosome X.13
The multiple myeloma cell establishes itself in the bone marrow by adhering to stromal cells and inhibiting osteoblastic activity and osteocalcin production. Adhesion of the multiple myeloma cell stimulates production of interleukin-6 (IL-6), a paracrine and autocrine growth factor for the multiple myeloma cell. IL-6 is one of several osteoclast-activating factors produced as a result of the multiple myeloma cell–stromal cell interaction. Increased osteoclastic activity plus inhibited osteoblastic activity results in osteoporosis, painful lytic lesions and hypercalcemia.14
B cells, T cells and macrophages undergo immunologic alterations related to marrow invasion. Such alterations confer an increased susceptibility to infections, particularly those caused by pneumococcus, tetanus and diphtheria organisms.15
The multiple myeloma cell clone produces an excess of monoclonal (M proteins) and free light chain proteins. The M proteins may be recognized as IgA, IgD, IgG, IgE or IgM, depending on their heavy chain class. This excess of M proteins is responsible for the hyperviscosity syndrome, which interferes with fibrin aggregation and platelet function.
The light chain proteins may be designated as kappa or lambda. They may precipitate and deposit, producing organ damage. The organ most commonly affected is the kidney. When these monoclonal light chains appear in the urine, they are called Bence Jones proteins. Bence Jones proteins may provoke a diversity of renal lesions, depending on the intrinsic physical or chemical properties of the light chain.16 Histopathologic findings range from no observable lesions to grossly abnormal features of glomerulosclerosis, cast nephropathy, acute tubulopathy, interstitial nephritis or infiltration of arterioles. The characteristic “myeloma kidney,” caused by cast nephropathy, occurs in about 28 percent of patients with multiple myeloma.17 Myeloma kidney results from toxicity to the distal nephron caused by an inflammatory response around the cast.16 Hypercalcemia, dehydration and use of nonsteroidal anti-inflammatory drugs (NSAIDs) may promote or worsen nephron obstruction by multiple myeloma casts.
Clinical Manifestations
The clinical presentation of multiple myeloma seems to be changing, probably as a result of diagnostic services that detect the disease in earlier stages. Thirty percent of new cases are diagnosed incidentally during evaluation for seemingly unrelated problems.18 A pathologic fracture is the presenting feature in 30 percent of cases.18
Two thirds of patients complain of bone pain, frequently located in the back, long bones, skull and pelvis. Patients with multiple myeloma commonly present with lower back pain. This may pose a diagnostic dilemma for the clinician trying to differentiate multiple myeloma from benign diseases of the spine. In 1994 the Agency for Health Care Policy and Research defined a number of “red flags” to be identified in the assessment of patients with acute lower back pain.19 Multiple myeloma should be considered as a diagnosis in patients over 50 years of age with back pain persisting more than one month if one or more red flags (Table 1) are identified.
| Age over 50 years |
| Pain that is worse in supine position |
| Pain that is worse at night or awakens patient from sleep |
| Pain with a band-like distribution around the body |
| Pain that is not relieved with conventional methods (i.e., rest, nonsteroidal anti-inflammatory drugs, acetaminophen [Tylenol]) |
| Associated constitutional symptoms (fever, weight loss, dehydration) |
| Progressive neurologic deficit in lower extremities |
In addition to pain, patients may complain of nonspecific constitutional symptoms related to hyperviscosity and hypercalcemia (Tables 2 and 3). The physician may notice a chronic, unresolving or incapacitating infection, commonly respiratory. Paresthesia and sensory loss may indicate neurologic damage caused by hyperviscosity, spinal cord compression or amyloid deposition.
| Pain in the lower back, long bones or ribs | |
| Generalized malaise | |
| Infections | |
| Fever | |
| Bleeding | |
| Symptoms of hypercalcemia | |
| Nausea | |
| Fatigue | |
| Thirst | |
| Symptoms of hyperviscosity | |
| Headaches | |
| Bruising | |
| Ischemic neurologic symptoms | |
| Other neurologic symptoms | |
| Peripheral neuropathy | |
| Meningitis | |
| Grade 0 | Normal radiograph |
| Grade 1 | Extensive osteoporosis or osteopenia |
| Grade 2 | Osteolysis |
| Grade 3 | Pathologic fracture |
On examination, local cavitation may be found in areas of osteoclastic activity, especially on the skull. The clinician may identify these cavitations as “soft spots” or even “holes” on the skull. Examination of the eyes may show exudative macular detachment, retinal hemorrhage or “cotton-wool” spots.20 There may be amyloid deposition on the tongue, causing macroglossia. Cardiovascular evaluation may reveal cardiomegaly related to deposition of immunoglobulin. A positive Tinel sign, Phalen sign, or both, indicates carpal tunnel compression, which is commonly due to amyloid deposition.
Diagnosis
Routine laboratory work-up may show pancytopenia, abnormal coagulation, hypercalcemia, azotemia, elevated alkaline phosphatase and erythrocyte sedimentation rate, and hypoalbuminemia. Examination may reveal proteinuria, hypercalciuria, or both. Urine dipstick tests may not indicate the presence of Bence Jones proteinuria. All patients with suspected multiple myeloma require a 24-hour urinalysis by protein electrophoresis to determine the presence of Bence Jones proteinuria and kappa or lambda light chains.
In patients with renal involvement, Fanconi syndrome may be the presenting manifestation.16 This syndrome is characterized by aminoaciduria, hyponatremia, hypoglycemia associated with glucosuria, low anion gap (less than 5 mEq per L) and hyperchloremic metabolic acidosis.
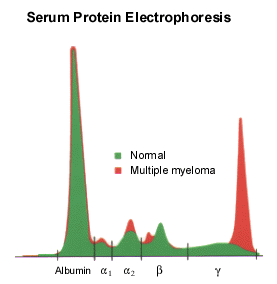
Serum protein electrophoresis will identify an M protein as a narrow peak or “spike” in the γ, β or α2 regions of the densitometer tracing (Figure 1). If multiple myeloma is strongly suspected and electrophoresis is “normal,” serum immunofixation may be more sensitive in identifying a small M protein.21 Only rarely is there no monoclonal proliferation (i.e., nonsecretory multiple myeloma), which occurs in 1 percent of patients.
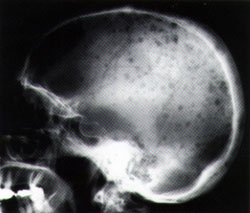
Skeletal lesions are observed on simple radiographs in 80 percent of cases22 (Table 4, Figures 2a and 2b). The most common finding is diffuse osteopenia. The radiographic findings often relate to vertebral compression fractures. Bone scintigraphy is used as a single test to screen for other bone lesions.
| Disease | Distinctive features |
|---|---|
| MGUS | M protein < 3 g per dL (30 g per L), < 10% plasma cell in bone marrow, no urine M protein, no lytic lesions, anemia, hypercalcemia or renal disease |
| SMM | M protein > 3 g per dL (30 g per L), > 10% plasma cell in bone marrow, no lytic lesions, no anemia, hypercalcemia or renal disease |
| PCL | > 20% plasma cell in peripheral blood, small levels of M protein, few bone lesions, few hematologic disturbances; younger population |
| SP | Only one tumor with no other bone lesions, urine or serum abnormalities |
| WM | IgM M protein, hyperviscosity, hypercellular bone marrow with extensive infiltration by lymphoplasma cells |
| HCD | M protein with an incomplete heavy chain lacking a light chain |
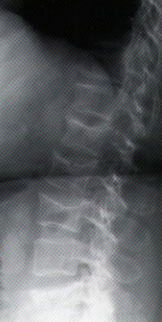
Magnetic resonance imaging (MRI) is highly accurate in depicting multiple myeloma tumors.22,23 When a vertebral lesion is suspected, MRI can identify early epidural involvement and spinal cord compression and assess tumor mass. Radiographic improvement occurs in 30 percent of treated patients, enabling the efficacy of therapy to be evaluated.
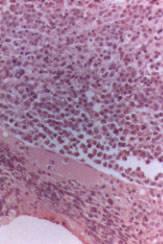
A 10 to 15 percent plasma cell involvement identified by bone marrow aspiration or biopsy is required for definitive diagnosis of multiple myeloma (Figure 3). Plasma cell morphology enables histologic classification of the disease, an important prognostic factor.
Differential Diagnosis
Connective tissue disorders, chronic infections and skeletal metastasis may have the same clinical presentation as multiple myeloma. Other causes of monoclonal gammopathy include monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), plasma cell leukemia, solitary plasmacytoma, Waldenstrom macroglobulinemia, heavy chain disease and amyloidosis (Table 4).
The incidence of monoclonal gammopathy in the healthy geriatric population is as high as 8 percent.24 Patients with MGUS require follow-up, since about 2 percent develop multiple myeloma or another malignant monoclonal gammopathy per year.25 Most patients with SMM do not progress to multiple myeloma, and their follow-up is stable. Forty percent of patients with multiple myeloma develop plasma cell leukemia. Overt multiple myeloma develops in 55 percent of patients with solitary plasmacytoma within three to four years.21
Amyloidosis may be primary and unrelated to other proliferative diseases or secondary and associated with multiple myeloma. In patients with amyloidosis, deposition of monoclonal light chain fibrils is widespread, particularly in the heart and kidneys, resulting in organ failure. Congo red staining of abdominal fat tissue will identify the fibrils in 80 percent of cases. A rectal biopsy that includes tissue from the submucosa has a sensitivity of 75 percent in the detection of amyloidosis.
Prognosis
Multiple myeloma has a general median survival of three years. The most significant factor among all parameters is beta2-microglobulin levels, which correlate with the Durie and Salmon clinical staging system for assigning prognosis26 (Table 5). Beta2-microglobulin is a light chain protein that is elevated in all lymphoproliferative diseases. Levels above 6 mg per L carry a shorter survival.24
| Stage I— Multiple myeloma cell mass < 0.6 cells × 1012 per m2 | |
| All of the following features are present: | |
| 1. Hemoglobin value > 10 g per dL (100 g per L) | |
| 2. Serum calcium value normal (< 12 mg per dL [2.99 mmol per L]) | |
| 3. Normal bone structure on radiographs (grade 0) or solitary plasmacytoma only | |
| 4. Low M-component production rates (IgG value < 50 mg per dL [0.5 g per L], IgA value < 30 mg per dL [0.3 g per L], urine light chain M component on protein electrophoresis < 4 g per 24 hours) | |
| Stage II—Multiple myeloma cell mass 0.6 to 1.2 cells × 1012 per m2 | |
| Features fitting neither stage I or stage III | |
| Stage III—Multiple myeloma cell mass > 1.2 cells × 1012 per m2 | |
| One or more of the following features are present: | |
| 1. Hemoglobin value < 8.5 g per dL (85 g per L) | |
| 2. Serum calcium value > 12 mg per dL (2.99 mmol per L) | |
| 3. Advanced lytic bone lesions (grade 3) | |
| 4. High M-component production rates (IgG value > 70 mg per dL [0.7 g per L], IgA value > 50 mg per dL [0.5 g per L], urine light chain M component on protein electrophoresis > 12 g per 24 hours) | |
| Subclassification: | |
| A. Relatively normal renal function (serum creatinine < 2 mg per dL [176 μmol per L]) | |
| B. Abnormal renal function (serum creatinine >2 mg per dL [176 μmol per L]) | |
Serum C reactive protein also may be used to determine prognosis in patients with multiple myeloma. When used with beta2-microglobulin, three risk groups can be identified: (1) patients with both parameters low and a median survival of 54 months; (2) patients with only one parameter elevated and a median survival of 27 months; and (3) patients with both parameters elevated and a median survival of six months.24
Another important parameter is the type of multiple myeloma histology observed on bone biopsy: plasmocytic, mixed cellular or plasmoblastic. Median survival time is approximately 39.7 months for patients with the plasmocytic type, 16.1 months for patients with the mixed cellular type and 9.8 months for patients with the plasmoblastic type.
Treatment
Treatment is divided into three phases: induction, maintenance, and relapse and reinduction.
Standard therapy for multiple myeloma includes alkylating agents administered with prednisone. The most commonly used alkylating agent is melphalan (Alkeran). Melphalan, 9 mg per m2, is given orally with 100 mg of prednisone on days 1 through 4. Courses of therapy are repeated at four- to six-week intervals for at least one year.
Good clinical response is a 50 percent reduction in the measured M protein levels. Complete remission is said to occur when the M protein cannot be identified by immunofixation and the bone marrow appears normal on biopsy.24 Up to 60 percent of patients respond to therapy with melphalan and prednisone, but only 3 percent have complete remission, and cure is extremely rare. After initial treatment, a three-month follow-up is indicated to plan further treatment. Bence Jones proteins and serum M proteins are expected to be reduced by 50 percent with this therapy. Melphalan with prednisone is also used as treatment for amyloidosis, but only 20 to 25 percent of patients respond to treatment.
The most important side effect of treatment with melphalan and prednisone is neutropenia or pancytopenia related to bone marrow suppression. Patients must be monitored weekly during treatment with melphalan. Pulmonary fibrosis and skin hypersensitivity have also been reported with melphalan therapy. Patients should be advised to contact their physician if they notice bleeding, fever, persistent cough or a skin rash. Women undergoing treatment should be advised to avoid pregnancy.
Other treatment modalities include alfa interferon (Intron A), combination chemotherapy, radiotherapy and stem-cell marrow transplantation. Alfa interferon reduces growth of myeloma colonies and the plasma cell labeling index in vitro, and prolongs the plateau phase. Alfa interferon can be used as monotherapy or together with melphalan and prednisone during the induction phase of treatment. In addition, it appears that response to chemotherapy is better following use of alfa interferon.27
Some patients with stage III multiple myeloma respond to combinations of vincristine (Oncovin), cyclophosphamide (Cytoxan) and doxorubicin (Adriamycin). However, only a few randomized trials have shown a survival benefit using combination chemotherapy. Most trials have not found significant differences between combination chemotherapy and melphalan with prednisone.24
Local radiotherapy, decompression surgery, or both, may be necessary to treat lytic bone lesions, particularly those involving the spine with resulting cord compression.28 Radiation therapy is useful in the control of local pain from lytic lesions. Patients with advanced stage III multiple myeloma may benefit from monthly intravenous infusions of pamidronate (Aredia). A large randomized, double-blind study showed a reduction in skeletal events when this bisphosphonate was used as an adjuvant to chemotherapy.29,30 Pamidronate may not alter the overall length of survival but may be useful in preventing osteoporosis and pathologic fractures.
Finally, allogeneic stem-cell marrow transplantation is currently being offered to selected patients. On average, 33 to 58 percent of all patients receiving HLA–matched allografts achieve complete remission, and 30 to 50 percent of these patients remain disease-free three to six years after transplantation. Prolonged remission (up to 16 years without evidence of active disease) has been observed in some patients who received allografts.31 The overall rate of mortality after transplant approaches 20 percent. Graft-versus-host disease accounts for significant morbidity and mortality in most reports. Infection is also a common complication because the patient receives total body irradiation and/or chemotherapy in preparation for the transplant. Most patients in whom transplant has been successful are less than 60 years of age and are responsive to chemotherapy. Most patients with multiple myeloma do not fulfill these criteria and may not be good candidates for transplantation. The benefit of bone marrow transplantation in multiple myeloma is still considered controversial by some experts.