
Am Fam Physician. 1999;59(7):1899-1908
Chronic critical limb ischemia is manifested by pain at rest, nonhealing wounds and gangrene. Ischemic rest pain is typically described as a burning pain in the arch or distal foot that occurs while the patient is recumbent but is relieved when the patient returns to a position in which the feet are dependent. Objective hemodynamic parameters that support the diagnosis of critical limb ischemia include an ankle-brachial index of 0.4 or less, an ankle systolic pressure of 50 mm Hg or less, or a toe systolic pressure of 30 mm Hg or less. Intervention may include conservative therapy, revascularization or amputation. Progressive gangrene, rapidly enlarging wounds or continuous ischemic rest pain can signify a threat to the limb and suggest the need for revascularization in patients without prohibitive operative risks. Bypass grafts are usually required because of the multilevel and distal nature of the arterial narrowing in critical limb ischemia. Patients with diabetes are more likely than other patients to have distal disease that is less amenable to bypass grafting. Compared with amputation, revascularization is more cost-effective and is associated with better perioperative morbidity and mortality. Limb preservation should be the goal in most patients with critical limb ischemia.
Atherosclerosis underlies most peripheral arterial disease. Narrowed vessels that cannot supply sufficient blood flow to exercising leg muscles may cause claudication, which is brought on by exercise and relieved by rest. (For a review of the diagnosis and management of claudication, see the article by Santilli, et al., in the March 1996 issue of American Family Physician.1) As vessel narrowing increases, critical limb ischemia can develop when the blood flow does not meet the metabolic demands of tissue at rest. While critical limb ischemia may be due to an acute condition such as an embolus or thrombosis, most cases are the progressive result of a chronic condition, most commonly atherosclerosis.
Chronic critical limb ischemia is defined not only by the clinical presentation but also by an objective measurement of impaired blood flow. Criteria for diagnosis include either one of the following (1) more than two weeks of recurrent foot pain at rest that requires regular use of analgesics and is associated with an ankle systolic pressure of 50 mm Hg or less, or a toe systolic pressure of 30 mm Hg or less, or (2) a nonhealing wound or gangrene of the foot or toes, with similar hemodynamic measurements.2 The hemodynamic parameters may be less reliable in patients with diabetes because arterial wall calcification can impair compression by a blood pressure cuff and produce systolic pressure measurements that are greater than the actual levels.
Risk Factors
Chronic critical limb ischemia is the end result of arterial occlusive disease, most commonly atherosclerosis. In addition to atherosclerosis in association with hypertension, hypercholesterolemia, cigarette smoking and diabetes,3,4 less frequent causes of chronic critical limb ischemia include Buerger's disease, or thromboangiitis obliterans, and some forms of arteritis.5
Diabetes is a particularly important risk factor because it is frequently associated with severe peripheral arterial disease. Atherosclerosis develops at a younger age in patients with diabetes and progresses rapidly. Moreover, atherosclerosis affects more distal vessels in patients with diabetes; the profunda femoris, popliteal and tibial arteries are frequently affected, while the aorta and iliac arteries are minimally narrowed. These distal lesions are less amenable to revascularization. Atherosclerosis in distal arteries in combination with diabetic neuropathy contributes to the higher rates of limb loss in diabetic patients compared with nondiabetic patients.6,7
Clinical Presentation
The development of chronic critical limb ischemia usually requires multiple sites of arterial obstruction that severely reduce blood flow to the tissues.7,8 Critical tissue ischemia is manifested clinically as rest pain, nonhealing wounds (because of the increased metabolic requirements of wound healing) or tissue necrosis (gangrene).
Ischemic rest pain is classically described as a burning pain in the ball of the foot and toes that is worse at night when the patient is in bed. The pain is exacerbated by the recumbent position because of the loss of gravity-assisted flow to the foot. Ischemic rest pain is located in the foot, where tissue is farthest from the heart and distal to the arterial occlusions.1 Patients with ischemic rest pain often need to dangle their legs over the side of the bed or sleep in a recliner to regain gravity-augmented blood flow and relieve the pain. Patients who keep their legs in a dependent position for comfort often present with considerable edema of the feet and ankles.
Nonhealing wounds are usually found in areas of foot trauma caused by improperly fitting shoes or an injury. A wound is generally considered to be nonhealing if it fails to respond to a four- to 12-week trial of conservative therapy such as regular dressing changes, avoidance of trauma, treatment of infection and debridement of necrotic tissue.
Gangrene is usually found on the toes. It develops when the blood supply is so low that spontaneous necrosis occurs in the most poorly perfused tissues.
Diagnosis
The presence of rest pain can sometimes be difficult to discern in patients with other chronic leg pain, such as that caused by peripheral neuropathy. Labeling a wound as nonhealing can also be a subjective assessment. However, a number of physical findings and objective hemodynamic parameters can be used to substantiate a diagnosis of chronic critical limb ischemia. Typical physical findings include absent or diminished pedal pulses, shiny smooth skin of the feet and legs, and muscle wasting of the calves.
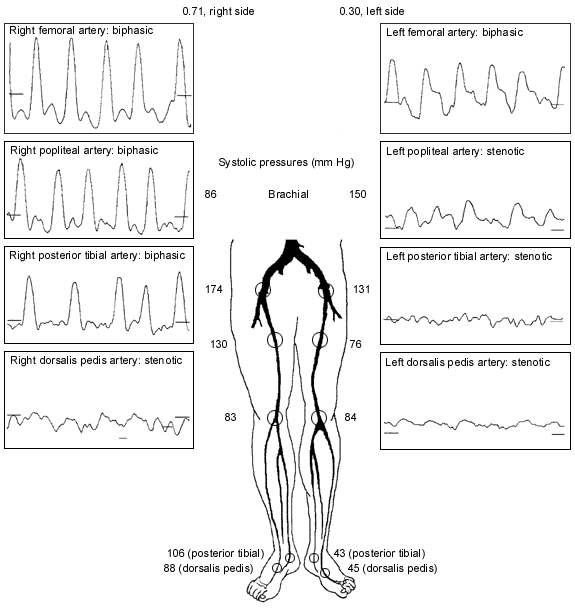
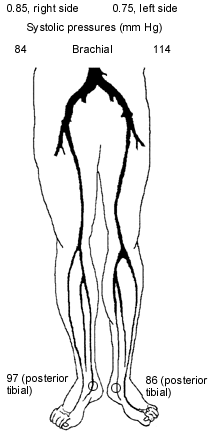
An objective measurement of blood flow is easily accomplished with the use of a hand-held Doppler probe and a blood pressure cuff.1 The cuff is inflated until the pulse distal to the cuff is no longer heard by Doppler. The cuff is then slowly deflated until the pulse is again detected. This measurement is recorded as the systolic pressure. As previously mentioned, an ankle systolic pressure of 50 mm Hg or less or a toe systolic pressure of 30 mm Hg or less suggests the presence of critical limb ischemia.
Another widely used parameter is the ankle-brachial index, which is a ratio of the systolic pressure at the dorsalis pedis or posterior tibial artery divided by the systolic pressure at the brachial artery. Patients with claudication typically have an ankle-brachial index of 0.5 to 0.8, while patients with critical limb ischemia usually have an ankle-brachial index of 0.4 or less.9,10
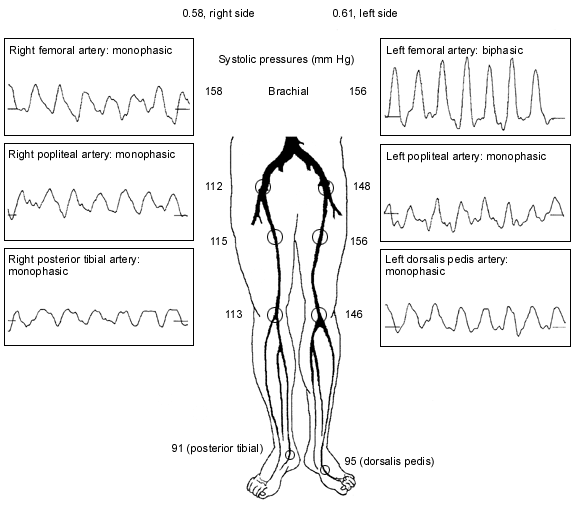
Vascular laboratories also use Doppler probes to measure the pulse volume waveform at segmental locations in the leg arteries. A change in the Doppler waveform from triphasic to biphasic to monophasic and then stenotic waveforms can identify sites of arterial blockage.
Differential Diagnosis
Ischemic rest pain may be confused with night cramps, arthritis or diabetic neuropathy. Night cramps occur in the calf muscles; they usually awaken the patient from sleep and are relieved by massaging the muscle, by walking or by using antispasmodic agents. Patients with arthritis of the metatarsal bones may have pain in the foot. This pain is often experienced at night and may be relieved by standing. The distinguishing characteristic of arthritic pain is that it usually occurs intermittently and at sporadic intervals, whereas ischemic rest pain consistently occurs after a specific interval of recumbency.
Diabetic neuropathy may also present with pain in the foot and is occasionally associated with diminished pulses and trophic skin changes. This pain, however, is not steadfastly associated with recumbency. The other features of diabetic neuropathy, such as loss of light touch (i.e., the monofilament test) and decreased vibratory sense, can also serve as distinguishing characteristics.
Conservative Treatment
Risk factor modification, including smoking cessation, blood pressure control, good glycemic control and reduction of lipid levels, should be instituted. Antiplatelet therapy with aspirin has been shown to substantially decrease the risk of myocardial infarction, stroke and death in patients with peripheral vascular disease and also reduces the rate of arterial reocclusion after angioplasty or bypass grafting.11
Ischemic Rest Pain
Patients with ischemic rest pain should be given pain medication as necessary, and any underlying systemic cause of inadequate blood flow, such as cardiac failure, should be corrected. If pain persists after four to eight weeks of conservative therapy with pain medication and interventions to optimize the patient's overall condition, the possibility of operative intervention should be explained to the patient, including the risks and benefits of the procedure.
Surgical intervention includes revascularization and amputation. If the patient wants to undergo revascularization and is an acceptable operative candidate, arteriography is often performed for further evaluation and planning of revascularization. Some centers are utilizing magnetic resonance angiography as an alternative or supplement to arteriography to minimize the risk of dye exposure.12
Nonhealing Wounds
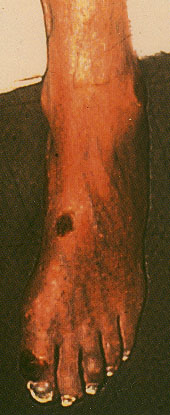
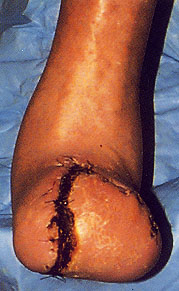
Patients with nonhealing wounds or gangrene should be evaluated for the presence of infection. Infected wounds require antibiotic therapy, surgical debridement, or both. Conservative therapy includes teaching the patient ways to avoid trauma to the wound site, including the wearing of properly fitting shoes. Dressings should be changed frequently; the patient should be seen weekly until the wound heals (Figures 1a through 1e).
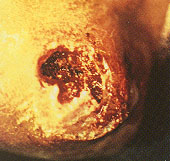
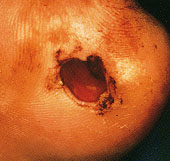
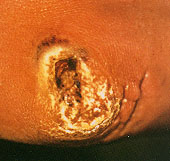
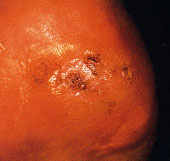
Further intervention may be required if conservative therapy does not lead to improvement, as indicated by increasing wound size, persistent or spreading infection or no evidence of healing after four to eight weeks. Progressive gangrene, rapidly enlarging wounds and continuous ischemic rest pain unrelieved by dependency are each unstable conditions that can rapidly lead to limb loss and require urgent intervention. However, many patients with critical limb ischemia have a stable or slowly progressive presentation. Review of the data reveals that patients with chronic critical limb ischemia have a three-year limb loss rate of about 40 percent.13–16 This suggests that a substantial proportion of patients with critical ischemia are not at risk of imminent limb loss.
Operative Intervention
REVASCULARIZATION
While carefully designed conservative therapy can benefit many patients with critical limb ischemia, the severe nature of their disease may lead to consideration of operative intervention. Surgical interventions include revascularization or amputation. If the patient wants to undergo revascularization and is an acceptable operative candidate, arteriography is often performed for further evaluation and planning of revascularization. At some centers, magnetic resonance angiography is used as an alternative or supplement to arteriography to minimize the risk of dye exposure.12 Limb preservation by means of revascularization is cost-effective, leads to a better quality of life for most patients and is associated with lower perioperative morbidity and mortality than amputation. Limb preservation should be the goal in most patients with chronic critical limb ischemia.13,14
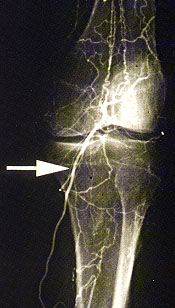
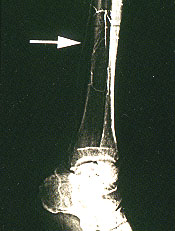
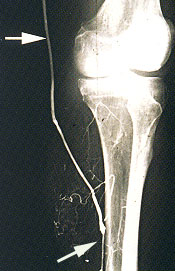
The feasibility of revascularization is determined by the arteriographic findings as well as the availability of a bypass conduit (Figures 2a through 2h). Angioplasty or stent placement, or both, is most successful with short, proximal lesions, such as those in patients with claudication, but is unlikely to be the only treatment necessary in the setting of critical limb ischemia because of the multilevel nature of the arterial occlusive disease. The ideal bypass conduit is the greater saphenous vein, but other conduits include the lesser saphenous veins, the arm veins or a prosthetic conduit. In most surgical series, three-year bypass patency rates of calf arteries range from 40 percent for prosthetic bypasses to 85 percent for saphenous vein bypasses.17–21 In comparison, studies of conservative therapy have demonstrated a 25 to 49 percent success rate with nonhealing wounds and a 50 to 80 percent rate of improvement in ischemic rest pain.13–16
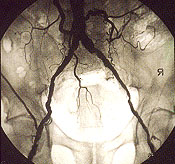
Primary Amputation
Primary amputation may be indicated in certain patients, such as those with extensive tissue necrosis, life-threatening infection or lesions not amenable to revascularization. The decision to monitor the patient's condition with watchful waiting and conservative management or to perform revascularization or amputation depends on careful assessment of the attendant risks and benefits of surgery versus conservative management.
More importantly, it depends on the patient's interpretation of the invasiveness or appropriateness of the available options. Even patients unable to walk because of their condition may consider amputation inappropriate, and not all patients are motivated to do the work necessary for rehabilitation after amputation. If the decision is made to amputate, the level of amputation should be one that has the greatest likelihood of healing while giving the patient the maximal chance for functional rehabilitation.
Follow-up
Patients with chronic critical limb ischemia require lifelong follow-up for a number of reasons. After amputation or revascularization, patients require rehabilitation to hasten their return to maximal independence. Careful attention to nutritional status will assist in wound healing and recovery. In addition, bypass graft patency should be assessed frequently after revascularization. Some authorities recommend periodic surveillance with duplex ultrasonography every three months to maximize the chance that restenosis is identified early, when it is more amenable to repair.12
Four-year survival rates as low as 40 percent are reported in patients with critical limb ischemia, with the vast majority of deaths caused by coronary artery disease and cerebrovascular disease.17 Close follow-up of coronary artery disease and cerebrovascular disease may help extend life expectancy in these high-risk patients.