
Am Fam Physician. 1999;59(11):3140-3148
Angiotensin-II receptor antagonists (or blockers) are a newer class of antihypertensive agents. These drugs are selective for angiotensin II (type 1 receptor); unlike angiotensin-converting enzyme inhibitors, they do not inhibit bradykinin metabolism or enhance prostaglandin synthesis. Angiotensin-II receptor antagonists are well tolerated. Cough occurs much less often with these agents than with angiotensin-converting enzyme inhibitors, and they do not adversely affect lipid profiles or cause rebound hypertension after discontinuation. Clinical trials indicate that angiotensin-II receptor antagonists are effective and safe in the treatment of hypertension. Their use in congestive heart failure and renal disease is under investigation.
Since the first angiotensin-II receptor antagonists were introduced a few years ago, numerous clinical trials have been conducted on their use in patients with hypertension and their potential use in patients with congestive heart failure. The angiotensin-II receptor antagonists that have been labeled for use in hypertension by the U.S. Food and Drug Administration (FDA) are losartan (Cozaar), valsartan (Diovan), irbesartan (Avapro), candesartan (Atacand) and telmisartan (Micardis). Other angiotensin-II receptor antagonists currently under investigation include eprosartan, tasosartan and zolarsartan.1
Renin-Angiotensin-Aldosterone System
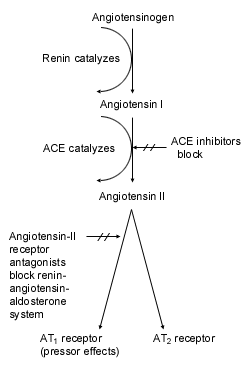
The renin-angiotensin-aldosterone system plays an integral role in the pathophysiology of hypertension because it affects the regulation of fluid volume, electrolyte balance and blood volume. Renin, an enzyme produced primarily by the juxtaglomerular cells of the kidney, catalyzes the conversion of angiotensinogen into an inactive substance, angiotensin I (A-I). Angiotensin-converting enzyme (ACE) then converts A-I to the physiologically active angiotensin II (A-II), which causes potent vasoconstriction, aldosterone secretion and sympathetic activation. All of these actions contribute to the development of hypertension.1–3
Two systems of nomenclature are used in reference to angiotensin-II receptor antagonists: one system employs Roman numerals, and the other is based on the amino acids that make up the A-I and A-II receptors (AT1 receptor and AT2 receptor).2 Angiotensin-II receptor antagonists act by binding to specific membrane-bound receptors that displace A-II from its type 1–receptor subtype (AT1). These drugs therefore function as selective blockers.3 AT-II pressor effects are mediated by AT1 receptors. These receptors are widespread in organs and tissues but are found predominately in vascular and myocardial tissue, the liver, the adrenal cortex (i.e., the zona glomerulosa tissue, which secretes aldosterone) and some areas of the brain.1–3
The effect of A-II is likely to be more completely blocked by the AT1 receptor antagonism of angiotensin-II receptor blockers than by ACE inhibitors. AT2 receptors are found in many tissues, including those of the adrenal medulla, uterus, ovary and other brain regions, but they are not known to be related to cardiovascular homeostasis.1,4 Angiotensin-II receptor blockers antagonize A-II–induced biologic actions, including smooth muscle contraction, sympathetic pressor mechanisms and aldosterone secretion.3 Thus, they inhibit the actions of A-II that are associated with hypertension.2–4 The mechanisms of action for ACE inhibitors and angiotensin-II receptor blockers are depicted schematically in Figure 1.
Specific Angiotensin-II Receptor Antagonists
The pharmacokinetic properties of the currently approved angiotensin-II receptor antagonists are summarized in Table 1,5–9,12 and dosage information is provided in Table 2.5–9 The antihypertensive effects of these drugs should become apparent within two to four weeks after the initiation of therapy. Greater blood pressure–lowering effect is achieved by adding a low-dose diuretic such as hydrochlorothiazide than by increasing the angiotensin-II receptor antagonist dosage.5–11 Hydrochlorothiazide provides blood pressure reduction through mechanisms such as mild natriuresis and vasodilation of blood vessels.
| Drugs | Time to peak concentration | Absolute bioavailability | Administration with food: area under the curve | Elimination half-life | Elimination altered renally | Elimination altered hepatical ly | Protein binding |
|---|---|---|---|---|---|---|---|
| Losartan (Cozaar) | 1 hour for losartan, 3 to 4 hours for its active metabolite* | Approximately 33% | Decreased 10% | 2 hours for losartan, 6 to 9 hours for its active metabolite* | No | Yes | 98.7 % |
| Valsartan (Diovan) | 2 to 4 hours | Approximately 25% | Decreased 40% to 50% | 6 hours | No | Yes | 95 % |
| Irbesartan (Avapro) | 1.5 to 2 hours | 60% to 80% | No effect | 11 to 15 hours | No | No | 90 % |
| Candesartan (Atacand)† | 3 to 4 hours | Approximately 15% | No effect | 9 hours | No | Yes | >99 % |
| Telmisartan (Micardis) | 0.5 to 1 hour | Approximately 42% to 58% | Decreased 6% to 20% | 24 hours | No | Yes | 99.5 % |
| Drugs | Dosage range | Recommended initial dosage* | Strengths available | Tablet/ capsule | Cost† |
|---|---|---|---|---|---|
| Losartan (Cozaar) | 25 to 100 mg once or twice daily | 50 mg once daily | 25, 50 mg | Unscored tablet | 50 mg once daily: $38 |
| Valsartan (Diovan) | 80 to 320 mg once daily | 80 mg once daily | 80, 160 mg | Capsule | 160 mg once daily: $36 |
| Irbesartan (Avapro) | 75 to 300 mg once daily | 150 mg once daily | 75, 150, 300 mg | Unscored tablet | 150 mg once daily: $36 |
| Candesartan (Atacand) | 8 to 32 mg once or twice daily | 16 mg once daily | 4, 8, 16, 32 mg | Unscored tablet | 16 mg once daily: $36 |
| Telmisartan (Micardis) | 40 to 160 mg once daily | 40 mg once daily | 40, 80 mg | ‡ | 80 mg once daily: $39 |
LOSARTAN
Losartan was the first angiotensin-II receptor antagonist to be introduced (1995). Compared with the parent drug, the active metabolite (EXP3174) has a longer half-life and antihypertensive effects that correlate more with plasma concentration.2 Double-blind studies have shown that losartan is well tolerated and as efficacious as enalapril and nifedipine for lowering blood pressure.13,14 The mean blood pressure reduction achieved with losartan in a dosage of 50 to 150 mg once daily is 5.5 to 10.5 mm Hg for systolic pressure and 3.5 to 7.5 mm Hg for diastolic pressure.5 One review of the efficacy and safety of losartan in the treatment of essential hypertension indicated a slowly developing response, with blood pressure becoming lower over several weeks of continued treatment.15
The starting dosage of losartan is 50 mg once daily. The duration of activity for a dose is 24 hours. Twice-daily dosing can be used if the antihypertensive effect measured at a trough is inadequate.5 However, a comparison of losartan in dosages of 100 mg once daily and 50 mg twice daily showed no significant difference in antihypertensive efficacy.16
A hydrochlorothiazide-losartan combination (Hyzaar) is also available. This combination drug contains 12.5 mg of hydrochlorothiazide and 50 mg of losartan. Some investigators advocate the use of this combination instead of escalation of a single drug, because dose-dependent adverse effects are less likely to occur.14 Dosing is once or twice daily.
VALSARTAN
Placebo-controlled trials have found valsartan to be both safe and effective for the treatment of hypertension.6,17 With valsartan taken in a dosage of 80 to 320 mg once daily, the mean reduction in diastolic blood pressure is 6 to 9 mm Hg, and the mean reduction in systolic pressure is 3 to 6 mm Hg.6 Studies have shown that valsartan is as effective as enalapril, lisinopril and amlodipine in the treatment of mild to moderate hypertension.18–20
The affinity of valsartan for the AT1 receptor is about 20,000 times greater than its affinity for the AT2 receptor.6 In comparison, the affinity of losartan for the AT1 receptor is about 1,000 times greater than its affinity for AT2 receptors.5 The clinical implication of receptor affinity is not yet clear.
Valsartan is also available as a combination product with hydrochlorothiazide (Diovan HCT). This combination drug contains 80 or 160 mg of valsartan and 12.5 mg of hydrochlorothiazide. With the addition of hydrochlorothiazide, blood pressure decreases even more (i.e., by 6 mm Hg systolic and 3 mm Hg diastolic). Dosing is once daily.
IRBESARTAN
In one study, 530 patients with mild to moderate hypertension were given placebo, losartan in a dosage of 100 mg per day or irbesartan in a dosage of 150 or 300 mg per day.21 After only one week of therapy, blood pressure trough reduction was significantly greater with irbesartan in a dosage of 300 mg per day than with losartan in a dosage of 100 mg per day.
Placebo-controlled trials have shown that irbesartan in a dosage of 150 to 300 mg per day lowers mean systolic blood pressure by 8 to 12 mm Hg and mean diastolic pressure by 5 to 8 mm Hg.7 Irbesartan has also been found to be as effective as enalapril and atenolol in reducing blood pressure.22 A combination product that contains both irbesartan and hydrochlorothiazide is being developed.
CANDESARTAN
Candesartan cilexetil has been shown to be effective for the treatment of hypertension. Candesartan itself is poorly absorbed after oral administration; the ester prodrug, candesartan cilexetil, improves bioavailability (Table 2). With oral administration of candesartan cilexetil, conversion to the active compound occurs rapidly and completely during gastrointestinal absorption.23 The affinity of candesartan for the AT1 receptor is more than 10,000 times greater than its affinity for the AT2 receptor.8
Candesartan is both safe and well tolerated in dosages of 8 to 32 mg per day. With these dosages, systolic blood pressure is reduced by 8 to 12 mm Hg and diastolic pressure is reduced by 4 to 8 mm Hg.8
Comparable reductions of diastolic blood pressure have been achieved with candesartan in a dosage of 8 mg per day and enalapril in a dosage of 10 mg per day.24 In one trial,25 significant reductions in mean sitting diastolic pressures occurred after 12 weeks of treatment with candesartan in a dosage of 8 or 12 mg per day and enalapril in a dosage of 10 mg per day (P < 0.01), but not with candesartan in a dosage of 4 mg per day (P = 0.074). The same study compared losartan in a dosage of 50 mg per day with candesartan in dosages of 8 and 16 mg per day.25 The 16-mg dosage of candesartan reduced diastolic blood pressure by an adjusted mean of 3.7 mm Hg more than the 50-mg losartan dosage.
TELMISARTAN
Telmisartan is the most recently labeled angiotensin-II receptor antagonist. Its affinity for the AT1 receptor is more than 3,000 times greater than its affinity for the AT2 receptor. Nonlinear pharmacokinetics yield a greater than proportional increase in plasma telmisartan concentrations with increasing dosages.9
The efficacy of telmisartan in the treatment of hypertension has been demonstrated in placebo-controlled trials. A three-month study of 440 patients showed that telmisartan in a dosage of 40, 80, 120 or 160 mg per day produced a slightly greater antihypertensive effect than enalapril in a dosage of 20 mg per day.10 In this study, diastolic blood pressure reductions with telmisartan ranged from 8.6 to 9.3 mm Hg, and systolic blood pressure reductions ranged from 10 to 11.9 mm Hg. The decreases in diastolic and systolic blood pressures for enalapril were 7.2 and 8.2 mm Hg, respectively.
Like the other angiotensin-II receptor antagonists, telmisartan has been shown to have a side effect profile similar to that of placebo. Clinical trials have demonstrated no rebound hypertension or first-dose orthostatic effect.11
Side Effects and Drug Interactions
The side effects of angiotensin-II receptor antagonists are comparable to those of placebo. Unlike ACE inhibitors, the angiotensin-II receptor antagonists are not significantly associated with cough.5–9 Prospective studies have shown that cough occurs in 7 to 15 percent (or more) of patients treated with ACE inhibitors.26,27 The accumulation of bradykinin and substance P, as well as the activity of kininase II, is thought to increase bronchial reactivity and the potential for development of the dry, persistent cough associated with ACE inhibitors.26,27 In addition, prostaglandins and leukotrienes may form from bradykinin and contribute to bronchial inflammation.26,27 Angiotensin-II receptor antagonists do not affect the activity of kininase II or the metabolism of kinins. In controlled trials using these drugs, the incidence of dry cough was comparable to that with placebo (i.e., about 1.5 to 3 percent.)5–9
Life-threatening angioedema has been associated with both ACE inhibitors and angiotensin-II receptor antagonists.27,28 In several reported cases, patients experienced angioedema with an ACE inhibitor and, when challenged with an angiotensin-II receptor antagonist, had the same effect at varying times of onset (i.e., hours to weeks or even months after starting treatment). The incidence of angioedema appears to be lower with angiotensin-II receptor antagonists than with ACE inhibitors. However, these agents have been in clinical use for a much shorter time than ACE inhibitors. Postmarketing surveillance should help to further define the overall incidence of angioedema in patients treated with the newer drugs.
Dizziness is a drug-related side effect that occurs in about 2 to 4 percent of patients taking angiotensin-II receptor antagonists.5–8 This side effect is occasionally associated with a first-dose hypotensive effect (fewer than 1 percent of patients).5–8 Thus, these agents should be started in a low dosage, which should then be titrated according to antihypertensive effect (Table 2).
Infrequent adverse effects with a possible causal association include nausea, headache, upper respiratory tract infection, back pain, fatigue, diarrhea, dyspepsia, nasal congestion, sinusitis and pharyngitis. Rarely, liver function tests and serum bilirubin concentrations may become elevated in patients treated with angiotensin-II receptor antagonists.5–9
When administered during the second and third trimesters of pregnancy, medications that act directly on the renin-angiotensin-aldosterone system have been associated with fetal and neonatal injury or death. Therefore, angiotensin-II receptor antagonists should not be used in pregnant women. In addition, none of these agents should be taken by patients who are breast-feeding because it is not certain how much drug is secreted in breast milk.5–9
Clinically significant drug interactions have been reported with losartan, which undergoes extensive first-pass metabolism by cytochrome P450 enzymes. Drugs such as ketoconazole (Nizoral) and troleandomycin (Tao) inhibit cytochrome P450 and thus may reduce formation of the active metabolite of losartan. The concomitant use of losartan and cimetidine (Tagamet) has been found to increase the bioavailability of losartan by 18 percent.29 Rifampin (Rifadin), an hepatic enzyme inducer, can cause a 20 percent reduction in the bioavailability of losartan and its active metabolite. The clinical implications of potential interactions with angiotensin-II receptor blockers are still uncertain.5,29
No significant drug interactions have been reported with valsartan, irbesartan or candesartan. A drug interaction can occur between telmisartan and digoxin (Lanoxin). In this interaction, the peak plasma concentrations of digoxin are increased, and the trough digoxin levels are elevated by 20 percent.11
RENAL EFFECTS
The AT1 receptor is found throughout the kidneys, including the renal vessels, afferent and efferent arterioles, and tubular and juxta-glomerular cells.1 Blockade of the renin-angiotensin-aldosterone system at the AT1 receptor site causes changes in renal hemodynamics and sodium excretion similar to those observed with ACE inhibitors.30
One study on the use of losartan in patients with kidney disease demonstrated an increase in renal plasma flow with a stable glomerular filtration rate.31 In this trial, serum potassium levels increased significantly, but uric acid levels did not change. Candesartan has been shown to have vasodilator effects within the renal circulation by decreasing renal vascular resistance.32
The occurrence of microalbuminuria has also been studied in patients treated with angiotensin-II receptor antagonists. In small studies, irbesartan, losartan and candesartan have been associated with a reduction in urine protein excretion.33–35 Angiotensin-II receptor antagonists are currently being investigated in patients with and without diabetes to evaluate renal effects and microalbuminuria.
Angiotensin-II receptor antagonists should be used cautiously in patients with renal dysfunction, and potassium levels should be monitored. These agents have the potential to elevate potassium concentrations, especially in patients who are also taking potassium-sparing diuretics.5–8,13 Potassium concentrations should also be monitored closely in patients with renovascular forms of hypertension, diabetes or hypoaldosteronism.1
In patients whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, such as those with severe congestive heart failure, the use of angiotensin-II receptor antagonists may result in oliguria, progressive azotemia and, rarely, acute renal failure or death.5–8,13 These drugs should not be used in patients with bilateral renal artery stenosis.
Congestive Heart Failure
Data are accumulating on the use of angiotensin-II receptor antagonists in the treatment of congestive heart failure. However, none of the drugs in this class has been labeled by the FDA for this indication. With the use of ACE inhibitors, AT-II production may be increased via a non-ACE–dependent pathway; this provides a theoretic rationale for possible combination therapy using an ACE inhibitor and an angiotensin-II receptor antagonist.36
Studies have compared the use of losartan and placebo in patients with New York Heart Association classes II through IV congestive heart failure and an ejection fraction of less than 40 percent.37,38 In these investigations, neurohumoral changes were noted in conjunction with reductions in serum aldosterone and norepinephrine activity. The Evaluation of Losartan in the Elderly (ELITE) study was a 48-week investigation conducted in 722 patients with congestive heart failure who were 65 years of age or older.39 In patients who were ACE-inhibitor naive, the effects of losartan in a dosage of 50 mg per day were compared with the effects of captopril in a dosage of 50 mg three times per day. No difference in the end point of increased serum creatinine was found between the two groups. The patients who were treated with losartan had fewer hospital admissions for heart failure than those who received captopril (9.4 and 13.2 percent, respectively; risk reduction of 32 percent). Because the study included only a small number of patients and these patients had relatively mild heart failure, further confirmation is required before the results can be seen as conclusive.
Although a number of trials have investigated the benefits of angiotensin-II receptor antagonists in the treatment of congestive heart failure, more data are needed on, for example, the role of these agents in patients with high filling pressures and more advanced congestive heart failure. The results of investigations currently in progress, including ELITE 2 and the valsartan heart failure trial, should shed light on these matters.
Place in Therapy
The sixth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-VI) stratifies patients into risk groups based on cardiovascular risk factors, target organ damage and clinical cardiovascular disease.40 Until equivalent long-term cardiac and renal protection has been demonstrated with the angiotensin-II receptor antagonists, ACE inhibitors remain the initial drugs of choice in patients with diabetes, heart failure or systolic dysfunction after myocardial infarction. At present, angiotensin-II receptor antagonists should be used cautiously (because of the possibility of renal impairment) in patients with congestive heart failure who cannot tolerate ACE inhibitors.
The advantages of angiotensin-II receptor antagonists include good efficacy with once-daily dosing and a safety and tolerability profile that is generally comparable to that of placebo. These drugs are lipid neutral, which may make their use advantageous in patients with hyperlipidemia.5–8,13 Losartan has been shown to have a neutral effect on glucose metabolism, insulin sensitivity and serum lipid concentrations in patients with mild hypertension.41 In clinical studies, candesartan has been found to have no appreciable effect on hemoglobin A1c level, serum glucose concentration or lipid profile.35
In general, the currently approved angiotensin-II receptor antagonists do not differ substantially with regard to blood pressure effects, and they all have a somewhat flat dose-response curve. However, better blood pressure–lowering effects are achieved when any of these agents is administered in combination with hydrochlorothiazide than when the dosage of the approved angiotensin-II receptor antagonist is increased. First-dose hypotension seldom occurs with this class of drugs.5–8 The angiotensin-II receptor antagonists do not change average heart rate, and no rebound hypertension occurs after these agents are discontinued.
Long-term trials are needed to evaluate cardiovascular end points, efficacy after myocardial infarction and dose response. Angiotensin-II receptor antagonists are not currently recommended as first-line therapy for congestive heart failure. However, data are accumulating to support their use in patients with congestive heart failure, especially patients who cannot tolerate ACE inhibitors. Combined therapy using ACE inhibitors and angiotensin-II receptor antagonists has also been proposed.42