
Am Fam Physician. 1999;60(1):99-108
Vision loss among the elderly is a major health care problem. Approximately one person in three has some form of vision-reducing eye disease by the age of 65. The most common causes of vision loss among the elderly are age-related macular degeneration, glaucoma, cataract and diabetic retinopathy. Age-related macular degeneration is characterized by the loss of central vision. Primary open-angle glaucoma results in optic nerve damage and visual field loss. Because this condition may initially be asymptomatic, regular screening examinations are recommended for elderly patients. Cataract is a common cause of vision impairment among the elderly, but surgery is often effective in restoring vision. Diabetic retinopathy may be observed in the elderly at the time of diagnosis or during the first few years of diabetes. Patients should undergo eye examinations with dilation when diabetes is diagnosed and annually thereafter.
The elderly population in the United States is increasing rapidly. By the year 2030, approximately 70 million Americans will be over 65 years of age. Loss of vision among the elderly is a major health care problem: approximately one in three elderly persons has some form of vision-reducing eye disease by the age of 65.1 Vision impairment is associated with a decreased ability to perform activities of daily living and an increased risk for depression.2 This article reviews the four most common causes of vision impairment in the elderly: age-related macular degeneration, glaucoma, cataract and diabetic retinopathy.3,4 Presenting symptoms of these four causes are summarized in Table 1.
Age-Related Macular Degeneration
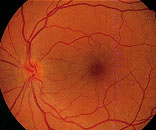
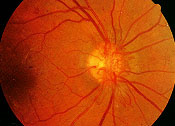
Age-related macular degeneration (AMD) is the leading cause of loss of vision in people over 65 years of age. AMD is characterized by degeneration of the macula, the area of the retina responsible for central vision (Figure 1). Risk factors for AMD include advancing age, family history of AMD and cardiovascular risk factors such as hypertension and cigarette smoking. AMD can be divided into two categories: nonexudative (or “dry”) AMD and exudative (or “wet”) AMD.
Approximately 90 percent of persons with AMD have the nonexudative form of the disease. Nonexudative AMD is the most common form of AMD, although it accounts for only 10 to 20 percent of cases of severe loss of vision in patients with AMD. Types of nonexudative AMD include drusen and geographic atrophy.
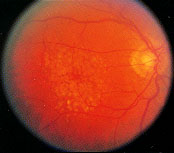
Drusen are localized deposits of extracellular material concentrated in the macula (Figure 2). The yellowish-white deposits tend to be symmetric in number and distribution, occurring in both eyes. When not associated with other changes, drusen usually do not disturb visual function; rarely, drusen alone may be associated with blurred or distorted vision (Table 1).
| Age-related macular degeneration | Blurred vision, image distortion, central scotoma, difficulty reading |
| Glaucoma | Visual field loss, blurred vision (late) |
| Cataract | Blurred vision, glare, monocular diplopia |
| Diabetic retinopathy | Blurred vision, floaters, visual field loss, poor night vision |
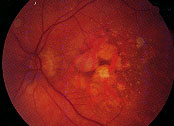
Geographic atrophy is responsible for nearly all cases of severe loss of vision associated with nonexudative AMD (Figure 3). Geographic atrophy is characterized by round or oval patches of atrophy of the retina, retinal pigment epithelium and underlying choroid. Over time, the patches may increase in size and number or may coalesce to form larger areas of atrophy. Geographic atrophy tends to be bilateral but may be asymmetric. Patients may complain of blurred or distorted vision, difficulty reading or driving, or increased reliance on brighter light or magnifying lenses to perform tasks requiring fine visual acuity.
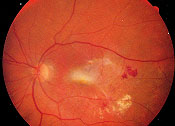
Approximately 10 percent of persons with AMD develop the exudative form of the disease. Exudative AMD accounts for 80 to 90 percent of cases of severe vision loss related to AMD. Exudative AMD is characterized by growth of abnormal vessels from the choroidal circulation to the subretinal space. These abnormal vessels leak fluid and blood in the macula, resulting in blurred or distorted central vision. Examination may reveal subretinal fluid, lipid exudate or blood; the choroidal neovascular membrane often has a grayish discoloration (Figure 4). The fibrovascular-choroidal neovascular complex may remain active for months to years. Eventually fibrosis develops, leaving a macular scar.
Most persons with nonexudative AMD have good vision throughout their lives. Loss of vision is the result of progressive geographic atrophy or, more commonly, the development of exudative macular degeneration. An annual eye examination, including dilation, is recommended to detect the signs of AMD in all persons 65 years of age or older. The elderly should be instructed about the symptoms of exudative AMD, including blurred vision, distortion or scotoma. Self-monitoring of vision is extremely important for those with AMD. This monitoring often includes the use of an Amsler grid to detect alterations in vision. An eye examination with dilation should be performed promptly if symptoms develop.
A higher dietary intake of foods rich in certain carotenoids may lower the risk of developing advanced or exudative AMD.5 Carotenoids may function as antioxidants to protect the retina. The two major carotenoids in the human retina are lutein and zeaxanthin. Foods rich in lutein and zeaxanthin include egg yolk, kiwi, squash and yellow squash, spinach, peas, honeydew melon, brussels sprouts, green beans, apples, corn, grapes, pumpkin, peppers, cucumber, orange juice, celery, scallions, broccoli and mango.6
The use of antioxidant vitamin and mineral supplements for the prevention or treatment of AMD is controversial.7–10 The Age Related Eye Disease Study, an ongoing multi-center randomized clinical trial, may help to clarify the role of antioxidant supplements for AMD. Recent studies have confirmed an increased risk of AMD among smokers; therefore, cigarette smoking should be strongly discouraged.11,12 Other factors associated with AMD, including hypertension and hypercholesterolemia, reinforce the importance of general systemic care in elderly patients with AMD.4,13
The prognosis of exudative AMD is guarded. Laser photocoagulation is effective in reducing the risk of severe loss of vision and limiting the extent of damage caused by exudative AMD.14 Unfortunately, only a minority of patients with exudative AMD are candidates for laser surgery. Low-vision aids, such as hand-held magnifiers, may be helpful in enhancing a person's ability to perform activities requiring fine visual acuity. Studies are currently under way to examine alternative treatment options for exudative AMD, including the following: antiangiogenic drugs to reduce the proliferation of choroidal new vessels; selective dye-enhanced laser photocoagulation; low-dose radiation therapy; and surgical intervention, including retinal transplantation/translocation.15
Although vision may become severely impaired, it is important to reassure patients with loss of vision related to AMD that complete blindness is not associated with this condition. Although AMD may result in loss of central vision, it almost never affects peripheral vision.
Glaucoma
Glaucoma comprises a group of disorders characterized by glaucomatous optic nerve damage and visual field loss. It is a significant cause of blindness in the United States and is the most common cause of blindness among black Americans. An estimated 1 million Americans over 65 years of age have experienced loss of vision associated with glaucoma, and approximately 75 percent of persons who are legally blind because of glaucoma are over the age of 65.16 The most prevalent form of glaucoma is primary open-angle glaucoma.
Primary open-angle glaucoma is responsible for approximately 10 percent of cases of blindness in the United States. Primary open-angle glaucoma affects men and women equally. Common factors associated with primary open-angle glaucoma include a family history of glaucoma, increasing age, high degree of myopia, hypertension and diabetes.
Primary open-angle glaucoma is a chronic, slowly progressive disorder. Persons with primary open-angle glaucoma are generally asymptomatic until late in the course of the disease, after suffering significant visual field loss. Primary open-angle glaucoma is bilateral but may be asymmetric. Examination reveals increased optic disc cupping (Figure 5), visual field loss (Figure 6), open and normal-appearing angles, and pretreatment intraocular pressures typically greater than 21 mm Hg (although intraocular pressures may be lower).
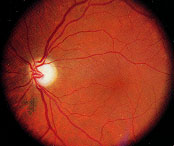
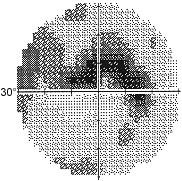
Medical therapy is usually the first line of treatment for primary open-angle glaucoma. Medications lower intraocular pressure by either reducing the secretion of aqueous humor by the ciliary body or enhancing aqueous outflow through the trabecular meshwork or alternative pathway. The six classes of intraocular pressure-lowering agents available are: beta-adrenergic antagonists, alpha2-adrenergic agonists, parasympathomimetic agents, carbonic anhydrase inhibitors and prostaglandin receptor agonists and hyperosmotic agents. Although most glaucoma medications are used topically, systemic side effects are possible (Table 2).
| Medication | Systemic side effects |
|---|---|
| Beta-adrenergic antagonists | Bradycardia, hypotension, heart failure, bronchospasm, syncope, headache, depression, impotence, gastrointestinal disturbance; may mask symptoms of hypoglycemia |
| Alpha2-adrenergic agonists | Headache, sleep disturbance, palpitations, arrhythmia, hypertension, gastrointestinal disturbance, dry mouth, fatigue |
| Parasympathomimetic agents | Headache, gastrointestinal disturbance, diaphoresis, dyspnea, bronchospasm, weakness, arrhythmia, hypotension, cholinesterase depletion |
| Carbonic anhydrase inhibitors | Taste perversion, anorexia, gastrointestinal disturbance, fatigue, depression, weakness, parasthesias, renal calculi, metabolic acidosis, blood dyscrasias, depressed libido |
| Prostaglandin receptor agonists | Muscle, joint, back, chest pain; rash |
Beta-adrenergic antagonists lower intraocular pressure by reducing the secretion of aqueous humor by the ciliary body. Noncardioselective beta-adrenergic antagonists include timolol (Timoptic), levobunolol (Betagan Liquifilm), metipranolol (Optipranolol) and carteolol (Ocupress). These medications have both beta1 and beta2 receptor blocking activity. Betaxolol (Betoptic) is a cardioselective beta-adrenergic agent that acts primarily on beta1 receptors. Because of the beta1 cardioselective nature of betaxolol, it may be used with fewer complications in patients with pulmonary disease.
Adrenergic agonists include epinephrine (Glaucon), dipivefrin (Propine), apraclonidine (Iodipine) and brimonidine (Alphagan). Epinephrine and dipivefrin act by increasing conventional and uveoscleral aqueous outflow. Apraclonidine and brimonidine are alpha2 agonists and act by decreasing aqueous secretion.
Parasympathomimetic drugs are divided into direct- and indirect-acting groups. Direct-acting agents such as pilocarpine (Pilopine HS) work in a manner similar to that of acetylcholine. In contrast, indirect agents act by inhibiting the enzyme acetylcholinesterase. Carbachol (Miostat) is a cholinergic agonist with both direct and indirect actions. All cholinergic agents increase aqueous outflow by contraction of the ciliary muscle. Because indirect agents suppress cholinesterase activity, potentially deleterious effects may occur if succinylcholine (Anectine), the skeletal muscle relaxant used in anesthesia, is administered to patients using these agents.
Acetazolamide (Diamox) and methazolamide (Neptazane) are systemic carbonic anhydrase inhibitors that act to reduce aqueous formation by inhibiting carbonic anhydrase activity in the ciliary body. The topical carbonic anhydrase inhibitors dorzolamide (Trusopt) and brinzolamide (Azopt) have proved to be viable alternatives to oral carbonic anhydrase inhibitors with fewer systemic side effects.17
The prostaglandin receptor agonist latanoprost (Xalatan) increases the uveoscleral outflow of aqueous humor. It has a long duration of action and can be administered once daily.
Hyperosmotic agents such as mannitol (Osmitrol), glycerin (Osmoglyn) and isosorbide (Ismotic) are used occasionally as short-term therapy to lower intraocular pressure. These agents act by creating an osmotic gradient between the blood and vitreous, thereby moving fluid out of the vitreous into the blood, with a resultant decrease in intraocular pressure.
Surgical intervention—including laser or incisional techniques—is considered when glaucoma cannot be adequately managed by medical therapy. Argon laser trabeculoplasty reduces intraocular pressure by increasing aqueous outflow. Surgical trabeculectomy is an incisional procedure performed to create a new channel for the flow of aqueous from the anterior chamber into the subconjunctival space. This procedure is often performed in conjunction with the use of antimetabolites such as mitomycin (Mutamycin) or fluorouracil (Efudex). These adjuncts act to decrease postoperative scarring and inflammation, thereby maintaining the patency of the filter.
Because primary open-angle glaucoma is associated with early peripheral visual field loss and affects central vision late in the disease process, the American Academy of Ophthalmology recommends that the elderly have comprehensive eye examinations every one to two years. In addition to evaluating intraocular pressure and optic discs, formal visual field testing is important to detect early glaucomatous visual field loss in those suspected of having glaucoma.
Cataract
Cataract is a common cause of vision impairment in the elderly and the most common cause of blindness worldwide.18 In the United States, the potentially blinding effect of cataract among the elderly is dramatically reduced because cataract surgery is readily available, effective and safe. The prevalence of cataract increases with age from less than 5 percent in persons under 65 years of age to approximately 50 percent in those 75 years of age and older.19 Exposure to ultraviolet light may contribute to the progression of cataract formation.20
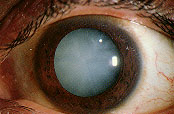
Although there is no universally accepted definition, “cataract” generally refers to lens opacities that interfere with vision function. Patients with visually significant cataracts may complain of blurred vision or glare. Cataract progression is typically slow, with gradual loss of vision over months to years. However, some types of cataract progress more rapidly.
Slit-lamp examination reveals varying degrees of opacification in one or several regions of the lens (Figure 7). Nuclear cataracts are associated with central lens opacification. The nucleus loses its transparency and may develop a yellow or brown discoloration. Cortical cataracts often consist of “radial spokes” extending from the periphery of the lens. Their effect on vision varies greatly depending on the location of the opacification relative to the visual axis. Posterior subcapsular cataracts are located in the posterior cortical layer and usually involve the central visual axis. They have a granular, plaque-like appearance. Posterior subcapsular cataracts may be associated with aging, trauma, systemic or topical corticosteroid use, intraocular inflammation or radiation exposure.
Cataract surgery is the most common surgical procedure covered by Medicare, with over 1 million procedures performed annually. This surgery should be considered when the cataract reduces vision function to a level that interferes with everyday activities. The mere presence of lens opacities—i.e., lens opacities not associated with decreased visual function—is not an indication for surgery in most instances. Cataract surgery is an outpatient procedure performed under local or topical anesthesia. Most cataract surgeries today are performed using phacoemulsification techniques.21 Phacoemulsification involves ultrasonic fragmentation of the lens into fine pieces, which are then aspirated from the eye.
The small incision size afforded by phacoemulsification facilitates more rapid vision rehabilitation than traditional cataract surgery.22 A lens implant is usually placed within the lens capsule but may be placed in front of the capsule or the iris if there is not enough capsular support (Figure 8). Opacification of the posterior capsule may develop in up to 50 percent of eyes with intraocular lens implants within three to five years. In these cases, Nd: YAG laser capsulotomy is a safe and effective procedure to clear the visual axis and restore visual function.
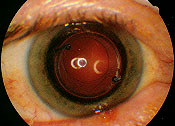
Over 90 percent of patients undergoing cataract surgery experience visual improvement and improved quality of life if there is no ocular comorbidity. Complications of cataract surgery are unusual and occur in less than 1 percent of surgeries. Potentially serious complications include glaucoma, bleeding, infection, vitreous loss, retinal detachment and loss of vision.
Diabetic Retinopathy
Diabetic retinopathy is the leading cause of new blindness among middle-aged Americans. It is also a significant cause of vision morbidity in the elderly population. The prevalence of diabetic retinopathy rises with increasing duration of diabetes. However, significant diabetic retinopathy may be observed in the elderly at the time of diagnosis or during the first few years of diabetes.23 Diabetic retinopathy is divided into two categories: nonproliferative and proliferative.
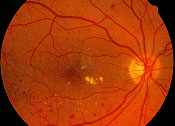
Nonproliferative diabetic retinopathy is characterized by abnormalities of the retinal circulation, including microaneurysms, intra-retinal hemorrhages, cotton-wool spots, retinal edema and exudates, and intraretinal microvascular abnormalities. The most common cause of visual loss in nonproliferative diabetic retinopathy is macular edema (Figure 9). Patients with macular edema may be asymptomatic or may complain of blurred or distorted central vision. Ophthalmoscopic examination reveals retinal thickening that is often associated with lipid exudate, microaneurysms and intraretinal hemorrhages.
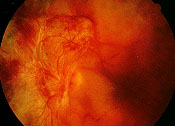
Proliferative diabetic retinopathy is characterized by the proliferation of newly formed blood vessels from the optic disc, retina or iris as the result of widespread retinal ischemia (Figure 10). The vitreous plays a critical role in the development and progression of proliferative diabetic retinopathy. It appears that the posterior vitreous provides a “scaffold” for the progression of neovascularization. As the posterior vitreous detaches, traction on the fibrovascular tissue increases. This may result in recurrent vitreous hemorrhage, traction retinal detachment, or both (Figure 11).
The Diabetes Control and Complication Trial24 confirmed the benefit of intensive blood glucose control in reducing the development and progression of diabetic retinopathy in patients with type 1 diabetes (formerly known as insulin-dependent diabetes). Similar results have been demonstrated in patients with type 2 diabetes (formerly known as non–insulin-dependent diabetes).25 Control of co-existing hypertension, hyperlipidemia, fluid overload states and renal failure may further reduce the adverse effects of diabetic retinopathy.26
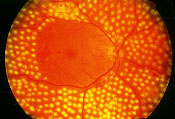
Surgical options are available for treatment of diabetic retinopathy. The Diabetic Retinopathy Study27 demonstrated that prompt panretinal laser photocoagulation performed in patients with high-risk proliferative diabetic retinopathy reduced the risk of severe vision loss by more than 50 percent (Figure 12). In eyes with clinically significant macular edema, the Early Treatment Diabetic Retinopathy Study28 showed that macular laser therapy significantly reduced the risk of visual loss. Panretinal photocoagulation for proliferative diabetic retinopathy and macular laser therapy for clinically significant macular edema are performed to reduce the risk of visual loss; early detection and treatment offer the best opportunity to reduce loss of vision associated with diabetic retinopathy. In patients with nonclearing vitreous hemorrhage or traction retinal detachment, pars plana vitrectomy may be used to reduce vision loss.
Since significant diabetic retinopathy may be present at the time of initial diagnosis of diabetes or shortly thereafter, elderly patients should undergo eye examinations with dilation at the time of diagnosis. Follow-up examinations should be performed annually in those with minimal or no retinopathy, or more frequently if significant retinopathy is detected.