
Am Fam Physician. 1999;60(8):2343-2352
See related patient information handout on flexible insulin regimens, provided by an AAFP staff patient education writer.
The management of type 1 diabetes mellitus (formerly known as insulin-dependent diabetes) has changed dramatically over the past 30 years. In particular, new insulin strategies have improved the ability to maintain near-normal glycemia. Factors such as onset, peak and duration of action can influence the ability of a particular insulin regimen to help control glucose levels. Patient factors, including individual variations in insulin absorption, levels of exercise and types of meals consumed, also influence the effectiveness of an insulin regimen. Rapid-acting insulin lispro is an ideal mealtime insulin. The premeal dose of insulin lispro can be adjusted based on the content of the meal and the patient's blood glucose level. Intermediate-acting and long-acting insulins should not be given to account for the content of a specific meal. Long-acting insulin can be administered once daily at bedtime or, ideally, twice daily in addition to another type of insulin. Patients with type 1 diabetes typically require an insulin dosage of 0.5 to 1.0 unit per kg per day. Newly diagnosed patients may have lower initial requirements because of continued endogenous insulin production. Flexible insulin regimens are based on predetermined actions in response to self-monitoring of blood glucose levels and carbohydrate intake.
Over the past 30 years, dramatic changes have occurred in the management of type 1 diabetes mellitus (formerly known as insulin-dependent diabetes).1–3 Insulin replacement strategies now stress the importance of administering smaller doses of insulin throughout the day. This approach allows insulin doses to be changed as needed to correct hyperglycemia, supplement for additional anticipated carbohydrate intake or subtract for exercise.
Although significant effort is required, it has become theoretically possible to maintain near-normal glycemia in most patients with type 1 diabetes. The 1993 report from the Diabetes Control and Complications Trial (DCCT) demonstrated that meticulous glycemic control is possible and reduces the occurrence of microvascular complications in patients who have type 1 diabetes.4
Pharmacology of Insulin
Several important factors affect the absorption of subcutaneously administered insulin and explain much of the unstable glycemia that occurs in patients with type 1 diabetes. The time it takes to absorb one half of an injected dose of insulin may vary by 25 to 50 percent among individual patients.5 For example, NPH insulin may have a duration of activity of 18 hours in one patient but an effective activity of only 9 or 10 hours in another patient.
| Factor | Effects |
|---|---|
| Site of injection | Abdominal injection (particularly if above the umbilicus) results in the quickest absorption; arm injection results in quicker absorption than thigh or hip injection. |
| Depth of injection | Intramuscular injections are absorbed more rapidly than subcutaneous injections. |
| Insulin concentration | U-40 insulin (40 units per mL) is absorbed more rapidly than U-100 insulin (100 units per mL). |
| Insulin dose | Higher doses have prolonged durations of action compared with lower doses. |
| Insulin mixing | Regular insulin maintains its potency and time-action profile when it is mixed with NPH insulin; however, mixing regular insulin with lente or ultralente insulin slows absorption and blunts the activity of regular insulin. |
| Exercise | Exercising a muscle group before injecting insulin into that area increases the rate of insulin absorption. |
| Heat application or massage | Local application of heat or massage after an insulin injection increases the rate of insulin absorption. |
| Type of insulin | Onset of action | Peak of action | Duration of action | Cost per vial for brand name insulin (generic insulin)* | Common pitfalls |
|---|---|---|---|---|---|
| Insulin lispro (Humalog) | 5 to 15 minutes | 1 to 2 hours | 4 to 5 hours | $ 28 | Hypoglycemia occurs if the lag time is too long or the patient exercises within one hour of administration; with high-fat meals, the dose should be adjusted downward. |
| Regular insulin (Humulin R) | 30 to 60 minutes | 2 to 4 hours | 6 to 8 hours | 22 (21) | Lag time is not used appropriately; the insulin should be given 20 to 30 minutes before the patient eats. |
| NPH insulin (Humulin N) | 1 to 3 hours | 5 to 7 hours | 13 to 18 hours | 22 (19 to 21) | In many patients, breakfast injection does not last until the evening meal; administration with the evening meal does not meet insulin needs on awakening. |
| Lente insulin (Humulin L) | 1 to 3 hours | 4 to 8 hours | 13 to 20 hours | 22 (18 to 21) | Zinc suspension binds with regular insulin, which loses its effect if it is left in the syringe for more than a few minutes. |
| Ultralente insulin (Humulin U) | 2 to 4 hours | 8 to 14 hours | 20 to 24 hours | 22 | Same as for lente insulin; in addition, peak of action is erratic in some patients. |
Another important factor that influences glycemia is the length of time between the administration of regular insulin or insulin lispro and the consumption of a meal (often called the “lag time”).2 This factor is perhaps the most underemphasized aspect of flexible diabetes therapy programs. In general, to ensure insulin availability during food consumption, regular insulin needs to be given 20 to 30 minutes before food consumption.6 Lag times need to be decreased when quicker-acting insulin lispro is used.
Although it is difficult to give exact recommendations on ideal lag times for the mealtime insulins (regular insulin and fast-acting insulin lispro), it is important to try to keep the timing of administration as consistent as possible. The lag time can be altered, depending on the level of premeal glycemia. Thus, when the blood glucose level is above the target range, it may be desirable to increase the lag time. Alternatively, the lag time should be decreased when premeal glycemia is below the target level, and insulin should be administered just before eating for premeal hypoglycemia.
SHORT-ACTING INSULIN: REGULAR INSULIN
The first insulin used, regular insulin is generally considered a mealtime insulin. However, with a peak effect in two to four hours after injection and a duration of action ranging from six to eight hours, regular insulin should also be considered a basal insulin because it still has significant activity after food is absorbed.
RAPID-ACTING INSULIN: INSULIN LISPRO
Insulin lispro is a fast-acting insulin analogue with a low capacity for self-aggregation in subcutaneous tissue. Regular human insulin has been shown to be equipotent to insulin lispro in terms of its binding to the insulin receptor and its effect on cellular glucose uptake.7
Although regular insulin has traditionally been classified as a short-acting insulin, insulin lispro is the first truly rapid-acting insulin. The quick action of insulin lispro makes it the ideal insulin for maintaining blood glucose levels below 180 mg per dL (10 mmol per L) for two hours after a meal, particularly when the meal contains foods that are relatively high in carbohydrates and low in fat.
When a low-carbohydrate, high-fat meal is to be eaten, the dose of insulin lispro should be adjusted downward to prevent hypoglycemia after a meal.8 Alternatively, when a high-fat meal is to be consumed, regular insulin may be substituted at that meal or a combination of regular insulin and insulin lispro may be used. Combined insulins, termed insulin “cocktails,” have become quite popular9 and have been shown to be useful in minimizing hyperglycemia after meals.10
To date, the greatest advantage documented for insulin lispro is the reduction of hypoglycemia as a result of the better matching of insulin and food absorption.11
INTERMEDIATE-ACTING INSULINS: NPH AND LENTE INSULINS
As noted in Table 2, the pharmacokinetic profile of NPH insulin shows more rapid onset and peak of action than have been appreciated by many physicians. These factors have several important clinical consequences. First, the risk of nocturnal hypoglycemia and fasting hyperglycemia is increased with insulin regimens using NPH insulin in combination with regular insulin and/or insulin lispro before mealtime.12 Second, the higher glucose levels seen in the early morning are caused by both the dissipation of insulin injected the previous night and the dawn phenomenon (a situation in which insulin resistance and greater insulin requirements are noted after 5 a.m.).12 It is clear that fasting hyperglycemia can be improved when NPH insulin is administered at bedtime instead of with the evening meal.13
Lente insulin appears to have a longer duration of action and a later peak of action than NPH insulin. Clinically, this is difficult to appreciate because of the normal variations in the two insulin preparations. Although some physicians prefer lente insulin to NPH insulin, it is important to be aware that lente and ultralente insulins contain excess zinc. When regular insulin is mixed with either NPH insulin or lente insulin, binding occurs with the zinc, and the regular insulin precipitates out of the solution. Consequently, if a mixture of regular and lente insulins remains in the syringe for more than a few minutes, the action of the regular insulin will be blunted.2
LONG-ACTING INSULIN: ULTRALENTE INSULIN
Ultralente insulin has a broad peak of action, ranging from eight to 10 hours in some studies and 12 to 16 hours in other studies.14 The duration of action for this insulin ranges from 20 to 24 hours.14 Ultralente insulin is best administered twice daily; if this insulin is administered once daily, it should be given at bedtime.2
Because of its prolonged peak and duration of action, ultralente insulin given before the evening meal improves fasting blood glucose levels compared with NPH or lente insulin administered at the same time.15 Reports in the literature are mixed about the suitability of ultralente insulin as a basal insulin.
Treatment Strategies
GENERAL CONSIDERATIONS
Most patients with C-peptide–negative type 1 diabetes require an insulin dosage of 0.5 to 1.0 unit per kg per day.2,16 Athletes and patients near ideal body weight generally require less insulin than sedentary or obese patients. In addition, patients with newly diagnosed type 1 diabetes generally have smaller initial insulin requirements because of continued endogenous insulin secretion. This is particularly relevant when type 1 diabetes presents in adulthood.17
In children and adults, insulin requirements immediately after diagnosis are usually in the range of 0.2 to 0.6 unit per kg per day. In these patients, glucose levels generally are less labile than they become when endogenous insulin production ceases completely. During this “honeymoon period,” or “remission,” it may be tempting to discontinue insulin administration altogether. However, discontinuation is not recommended because data suggest that the insulin may be altering the immune system to retard beta-cell destruction.18
ONCE-DAILY OR TWICE-DAILY INSULIN REGIMENS
Traditional once-daily or twice-daily insulin regimens are no longer recommended for most patients with type 1 diabetes who have little or no endogenous insulin production. However, twice-daily insulin injections may be effective for at least a short period in patients with newly diagnosed type 1 diabetes who are still producing a significant amount of insulin (Table 3).
| The total daily insulin dosage is 0.3 unit per kg of body weight. |
| Twice-daily administration of NPH insulin and regular insulin usually works adequately while endogenous insulin is still being produced. |
| Two thirds of the total daily insulin dose may be given 20 to 30 minutes before breakfast, and one third of the dose may be given 20 to 30 minutes before the evening meal. |
| As an estimate, NPH insulin and regular insulin can be given in a 2:1 ratio for the breakfast dose and a 1:1 ratio for the evening-meal dose. |
| As more complete insulin deficiency develops, this regimen becomes less effective. |
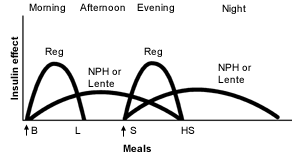
As noted in Table 2 and Figure 1,19 the peak of action for NPH or lente insulin creates several problems. The morning administration of a large dose of intermediate-acting insulin with regular insulin results in hyperinsulinemia at midday. If lunch is delayed even a short time in patients who maintain reasonable glycemia after breakfast, hypoglycemia ensues. To avoid this problem, many patients require a midmorning snack, even when lunch is not delayed. For patients on this regimen, a lack of hypoglycemia, even after a long delay to the midday meal, strongly suggests extremely high morning glucose levels. In many patients, especially those who are young and thin, the morning insulin tends to dissipate by late afternoon, resulting in high glucose levels before the evening meal. This particular issue was not as great a problem with bovine and porcine insulins,2 because they had longer durations of action than the human insulins used today.
Perhaps an even greater problem is maintenance of nocturnal glycemic control when an intermediate-acting insulin is injected with the evening meal (Figure 1).19 With the peaking of insulin occurring in the middle of the night, patients are predisposed to nocturnal hypoglycemia at a time when insulin requirements are at their lowest.12 Furthermore, when NPH or lente insulin is administered at the evening meal, the insulin often does not sustain its effect throughout the night, and fasting hyperglycemia occurs.
FLEXIBLE DIABETES THERAPY
For any flexible diabetes therapy to be effective, the basal and mealtime components must be identified. The basal component restrains hepatic glucose production, keeping it in equilibrium with tissues that are obligate glucose consumers (such as brain tissue). Mealtime insulin stimulates peripheral glucose uptake while inhibiting hepatic glucose output.
Basal insulin may be provided as (1) bedtime intermediate-acting insulin with or without morning intermediate-acting insulin, (2) ultralente insulin, usually administered twice daily, or (3) insulin pump therapy. Dosing can be determined only by assessing the blood glucose level after the insulin administered at mealtime has dissipated and food has been digested.
It is important to determine if the blood glucose level is maintained within the target range after breakfast. For example, if the glucose level before the midday meal is consistently elevated and the glucose level two hours after the meal is usually within the target range, the basal component in the morning may be too low. If the blood glucose level before the midday meal is above the target range and the glucose level two hours after the meal is also usually high, the mealtime insulin component is probably insufficient, or perhaps the basal and mealtime insulins need adjustment to a larger dose.
SPECIFIC FLEXIBLE INSULIN THERAPY PROGRAMS
NPH or Lente Insulin as the Basal Insulin. One popular solution to the problem of nocturnal insulin replacement is to delay administration of the intermediate-acting insulin until bedtime1,2,16 (Figure 2).19 With this approach, serum insulin levels before breakfast are higher (better matching insulin requirements), and nocturnal hypoglycemia should be less of a problem. When mealtime insulin is only administered twice (at morning and evening meals), mealtime flexibility is not significantly improved.
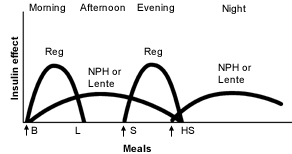
When regular insulin is used at mealtimes, some physicians prefer using bedtime intermediate-acting insulin as the only basal insulin.20 Because of regular insulin's relatively long duration of action, this approach may be effective in many patients. An injection of regular insulin is also given before the midday meal. This increases flexibility in that the timing of the midday meal may be more varied (because there will be no “peaking” NPH or lente insulin from breakfast), and a specific dose of insulin may be administered based on the blood glucose level, anticipated food consumption and planned exercise.
Other physicians prefer administration of a small dose of intermediate-acting insulin in the morning, a larger dose at bedtime and doses of insulin at mealtimes when needed16 (Figures 3 and 4).19 Many physicians believe that this results in “smoother” glycemia during the day. Perhaps more importantly, if insulin lispro is used as the mealtime insulin, a minimum of two injections of intermediate-acting insulin is required.21
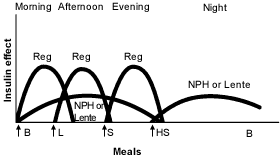
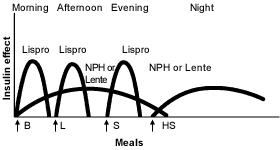
Ultralente Insulin as the Basal Insulin. Recently, ultralente insulin programs have become more popular2 (Figures 5 and 6).19 It is easiest to start ultralente insulin at approximately 50 percent of the total daily dosage, with one half of the dosage given with breakfast and the rest with the evening meal.
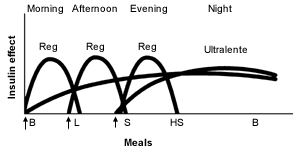
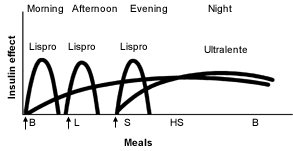
Persistent fasting hyperglycemia may be treated by increasing the dose of ultralente insulin that is given at the evening meal. However, this approach may not be effective in some patients, because nocturnal hypoglycemia may occur without any improvement in fasting hyperglycemia as a result of the dawn phenomenon.
Occasionally, patients do best with ultra-lente insulin given in the morning and NPH insulin administered as the other basal component at bedtime. The advantage of this regimen is that early-morning insulin resistance can be better targeted with the bedtime NPH insulin.
Action Plans
Any flexible diabetes therapy regimen is based on predetermined actions to be taken in response to self-monitoring of blood glucose levels or an unusual situation that can affect glycemia. Patients with type 1 diabetes need to be taught an action plan that takes into account all components necessary to correct glycemia. This plan would cover any changes in mealtime insulin dose, as well as lag time, food intake (especially carbohydrate consumption) and physical activity. Unfortunately, action plans for flexible diabetes therapy continue to be confused with sliding-scale insulin therapy, which refers to a retrospective correction of hyperglycemia with short-acting insulin without regard to caloric intake or physiologic insulin delivery.2,22
An insulin supplement is a temporary dose of regular insulin that is administered to prevent or correct a blood glucose level outside of the target range. A supplement may be used if premeal hyperglycemia is present, a particularly large meal is anticipated or usual physical activity is not going to be performed.
For supplementation using regular insulin or insulin lispro, the lag time must be considered. Regular insulin or insulin lispro should be used with caution at bedtime.12 With insulin regimens using NPH or lente insulin at breakfast, insulin supplements used before the midday meal often need to be more conservative than supplements given at other times of the day, because of significant activity from the intermediate-acting insulin.
Table 4 represents a sample plan for the administration of insulin lispro before meals in conjunction with a flexible diabetes therapy program using either twice-daily ultra-lente insulin or insulin pump therapy as the basal insulin component. A plan for the management of outpatient surgery in a patient with type 1 diabetes is presented in Table 5.
| Premeal blood glucose level, mg/dL (mmol/L) | Insulin dose | Lag time | Comments |
|---|---|---|---|
| < 50 (2.8) | Decrease by 2 units. | Inject during meal. | Include at least 10 g of simple carbohydrates in the meal. |
| 50 to 80 (2.8 to 4.4) | Decrease by 1 unit. | 0 | |
| 80 to 130 (4.4 to 7.2) | No change | 0 | |
| 130 to 150 (7.2 to 8.3) | Increase by 1 unit. | 0 | |
| 150 to 200 (8.3 to 11.1) | Increase by 2 units. | 10 minutes | |
| 200 to 250 (11.1 to 13.9) | Increase by 3 units. | 15 minutes | |
| 250 to 300 (13.9 to 16.7) | Increase by 4 units. | 20 minutes | Test for urinary ketones. |
| 300 to 350 (16.7 to 19.4) | Increase by 5 units. | 25 minutes | Test for urinary ketones; if levels are moderate or large, increase fluid intake and consider giving additional insulin. |
| 1. If possible, schedule the procedure early in the morning. | |
| 2. Basal insulin: | |
| If NPH insulin is used, give 50 percent of the usual dosage at the usual time. | |
| If ultralente insulin is used, give the entire daily dose at the usual time. | |
| 3. Mealtime insulin: | |
| For an early-morning procedure, withhold the insulin dose until after surgery unless the blood glucose level is higher than 200 mg per dL (11.1 mmol per L); in this situation, administer supplemental insulin.† | |
| For a late-morning or afternoon procedure, administer 50 percent of the usual dose of mealtime insulin at the usual time, with supplemental insulin given if the blood glucose level is higher than 200 mg per dL (11.1 mmol per L); ensure that the patient receives a dextrose infusion at 5 g per hour. | |
The continuing need for compensatory insulin supplements because of a pattern of unexplained blood glucose levels above the target range (e.g., continued fasting hyperglycemia) indicates that the relevant insulin component needs to be adjusted. Thus, adjustments are modifications in the basic insulin dose made in response to a pattern of glycemia over several days, assuming that no unusual circumstances are causing the blood glucose levels to be outside of the target range. Adjustments may apply to any type of insulin.
Carbohydrate Counting
With this method, patients are taught how to count the grams of carbohydrates they anticipate eating at a meal, and the dose of regular insulin or insulin lispro to be taken before the meal is then calculated based on the ratio of insulin to carbohydrate content. For example, 1 unit of insulin lispro may be appropriate for every 15 g of carbohydrate in a meal. This ratio needs to be individualized, and it may even vary for the same patient at different times of the day.
Although some patients can manage carbohydrate counting and insulin dose calculation, many patients find these tasks difficult. Others prefer to employ a carbohydrate goal (e.g., bread exchanges) and then use an insulin-to-carbohydrate ratio for additional (or less) carbohydrate. Regular insulin or insulin lispro may then be added (or subtracted) appropriately.
The major advantage of carbohydrate counting is that it adds yet another level of flexibility to the general issue of diet/calories and the relationship to insulin, perhaps the most important, least emphasized and least understood aspect of diabetes therapy. Historically, patients have made guesses (sometimes educated ones) about how much additional insulin to administer for added food. To a great extent, carbohydrate counting minimizes such estimations. Clearly, however, support and education from an experienced nutritionist are required for optimal results.
Modern-Day Therapy and the Diabetes Control and Complications Trial
In the landmark DCCT,25 patients randomized to the intensive therapy group could choose between a multiple daily insulin regimen (three or four injections per day) or insulin pump therapy. By the end of the study, 56 percent of patients chose multiple injections, and 42 percent chose pump therapy. Of the multiple-injection group, 44 percent used an ultralente insulin regimen, and the rest used NPH or lente insulin. However, many patients in the DCCT were still using animal-species insulins; thus, the absorption characteristics were different than those of the human insulins now used.
It is difficult to reflect back on the DCCT therapies of the 1980s and extrapolate to current diabetes therapy. Animal-species insulins are no longer available, and a plethora of insulin analogs will be introduced within the next three years. Insulin pump therapy has changed completely since the introduction of insulin lispro.
Perhaps a more important lesson from the DCCT is that 86 percent of patients in the intensive therapy group measured their blood glucose levels at least three times each day.25 Frequent self-monitoring of blood glucose levels is now required for a successful program. It is also quite clear that even mild hyperglycemia is dangerous for patients with type 1 diabetes or type 2 diabetes (formerly known as non-insulin–dependent diabetes) and that the goal glycosylated hemoglobin level should be less than 7 percent for most patients.