
Am Fam Physician. 2000;61(3):675-682
See related patient information handout on diet in children with high cholesterol, written by the authors of this article.
Accumulating evidence clearly shows that atherosclerosis begins in youth. The National Cholesterol Education Program (NCEP) has recommended that children at high risk of developing coronary artery disease as adults be screened so that those with elevated low-density lipoprotein (LDL) cholesterol levels can be treated, primarily by modification of diet. The initial approach to these youthful patients is to use the NCEP step I diet. This diet provides calories and nutrients that support normal growth and development, but limits saturated fat and total fat intake to no more than 10 and 30 percent of total calories, respectively, and cholesterol intake to no more than 100 mg per 1,000 kcal per day, to a maximum of 300 mg. If the goal of reducing the LDL cholesterol level to below 130 mg per dL (3.35 mmol per L) is not achieved, the more restrictive step II diet should be initiated. However, the step II diet may not provide sufficient calories and nutrients to support normal growth and development; therefore, trained nutritionists may be required to effectively manage a child on this diet.
Cardiovascular disease caused by atherosclerosis is the leading cause of death worldwide.1 Clinical evidence, epidemiologic data and postmortem studies provide convincing evidence that atherosclerosis begins in childhood. Children with homozygous familial hypercholesterolemia develop significant coronary heart disease (CHD) in the first decade of life and frequently die of myocardial infarction before 20 years of age.2–4 Serum cholesterol levels measured at 22 years of age are predictive of the risk for developing CHD over the next 30 to 40 years.5 Data from the Framingham Study6 show that cholesterol levels measured in young adults are predictive of CHD mortality 30 years later. In persons with familial hypercholesterolemia, there is a direct association between the duration and severity of hypercholesterolemia and extravascular lipid deposition in tissues.7
Scope and Epidemiology of the Cholesterol Problem
Epidemiologic data support the relationship between childhood and adult cholesterol levels,8–13 and a relationship between hyperlipidemia in the children and premature CHD in the parents.14,15 Pathologic findings have provided further evidence of the early development of atherosclerosis: young soldiers killed in the Korean War were found, with high frequency, to have advanced coronary artery lesions.16 Quantitative postmortem estimation17 of atherosclerosis in coronary arteries and aortas of children and young adults demonstrate a significant relationship between hyperlipidemia and the extent of atherosclerosis. In agreement with the accumulating evidence, it was recently demonstrated that cardiovascular risk factors in children are related to the severity of atherosclerosis found postmortem in such persons who died as young adults.18
Evidence of Need to Treat Hypercholesterolemia
Studies in adults provide evidence that reduction of serum cholesterol concentrations lowers the risk of developing CHD.19 The effectiveness of diet in reducing total and low-density lipoprotein (LDL) cholesterol levels is well established.20 Furthermore, studies in young children and adolescents have shown that when adequate calories and nutrients are provided, cholesterol-lowering diets do not impair growth or development.21–25
Based on these findings, the National Cholesterol Education Program (NCEP) Expert Panel on Blood Cholesterol in Children and Adolescents recommended dietary intervention to lower LDL cholesterol levels in adolescents and children at least two years of age with a family history of cardiovascular disease or parental hypercholesterolemia.26 Because dietary treatment is safe and often effective, physicians should be alert for opportunities to provide appropriate consultation and treatment or to refer patients and families to other health care professionals for consultation.
Approach to Drug Therapy of Hypercholesterolemia
The approach to drug therapy for hypercholesterolemia in childhood can also be found in the NCEP guidelines26 and in recent reviews27,28 of the subject. The recommendation in the original NCEP guidelines is to augment dietary treatment with the use of bile acid sequestrants in children more than 10 years of age who, after six months to one year of dietary treatment, have failed to lower their LDL cholesterol level to less than 190 mg per dL (4.90 mmol per L), or to below 160 mg per dL (4.10 mmol per L) if they have a family history of premature cardiovascular disease or two or more other risk factors (e.g., low high-density lipoprotein [HDL] levels, diabetes, obesity, hypertension, cigarette smoking and physical inactivity). A number of systemically acting lipid-lowering drugs have proved to be successful in secondary29,30 and primary31 prevention of CHD in adults. Because this article's focus is on dietary therapy and because of the lack of data on the safety of prolonged use of these medications in children, we will not further discuss their use in this article.
Which Children Should Be Screened?
The NCEP has recommended a selective approach in screening children for hypercholesterolemia. Screening should be performed in children more than two years of age who have a positive family history of premature cardiovascular disease or parental hypercholesterolemia.
Positive family history of premature cardiovascular disease is defined as a parent, grandparent or first-degree aunt or uncle who experienced one of the following before the age of 55: myocardial infarction, angina pectoris, peripheral vascular disease, cerebrovascular disease, sudden cardiac death or documented coronary atherosclerosis.
Parental hypercholesterolemia is defined as a total blood cholesterol level of 240 mg per dL (6.20 mmol per L) or higher. Cholesterol levels should also be obtained in patients who smoke, have diabetes mellitus, are obese or have hypertension.
Initial Evaluation and Follow-up
Lipid and lipoprotein measurements should be made by an experienced laboratory, especially one that participates in the voluntary quality control program of the Centers for Disease Control and Prevention (CDC). Studies should be performed twice, and an average of the two values should be used to determine further evaluation and treatment.
In children with a positive family history of premature cardiovascular disease, a fasting lipoprotein analysis (total cholesterol, total triglycerides, HDL and LDL cholesterol levels) should be obtained. In children, fasting means consuming nothing except water after midnight. Table 1 provides acceptable, borderline and high LDL cholesterol levels in children.
If there is no family history of premature cardiovascular disease, but the parents have hypercholesterolemia or other risk factors, a nonfasting total cholesterol level is a sufficient initial test. Acceptable levels are less than 170 mg per dL. If the total cholesterol is elevated above this level, a fasting protein analysis should be performed.
In a child with a high cholesterol level (total cholesterol or LDL cholesterol), screening tests for secondary causes of hypercholesterolemia (in particular, diabetes and diseases of the thyroid, liver and kidney) should be performed. Certain medications such as steroids, anticonvulsants and oral contraceptives can also be secondary causes. A more complete list of secondary factors can be found in the NCEP summary.26
Management
The goal of dietary treatment is to reduce LDL cholesterol levels. Table 1 provides the levels of total and LDL cholesterol at which dietary intervention is indicated in children and adolescents with a family history of hypercholesterolemia or premature cardiovascular disease. The recommended initial step to reduce cholesterol levels in children is the institution of a “heart-healthy” diet—one that is low in cholesterol and saturated fat and high in complex carbohydrates, and provides adequate energy for growth and the maintenance of a desirable weight.
AHA STEP I DIET
The American Heart Association (AHA) step I diet is equivalent to the NCEP step I diet and is well established for this purpose in adults and children more than two years of age.32 In a step I diet, no more than 30 percent of total calories come from fat, less than 10 percent of total calories come from saturated fat and dietary cholesterol is restricted to 100 mg per 1,000 kcal, not to exceed 300 mg per day. Many foods that are high in cholesterol are also high in saturated fat. A step I diet substitutes foods rich in monounsaturated and polyunsaturated fats for those rich in saturated fats. To provide effective dietary guidance, a complete dietary assessment is required. This is often difficult to accomplish in the typical office setting, but suggestions for estimating dietary intake and modifying the diet are noted below, as well as in Figure 1 and the accompanying patient information handout.
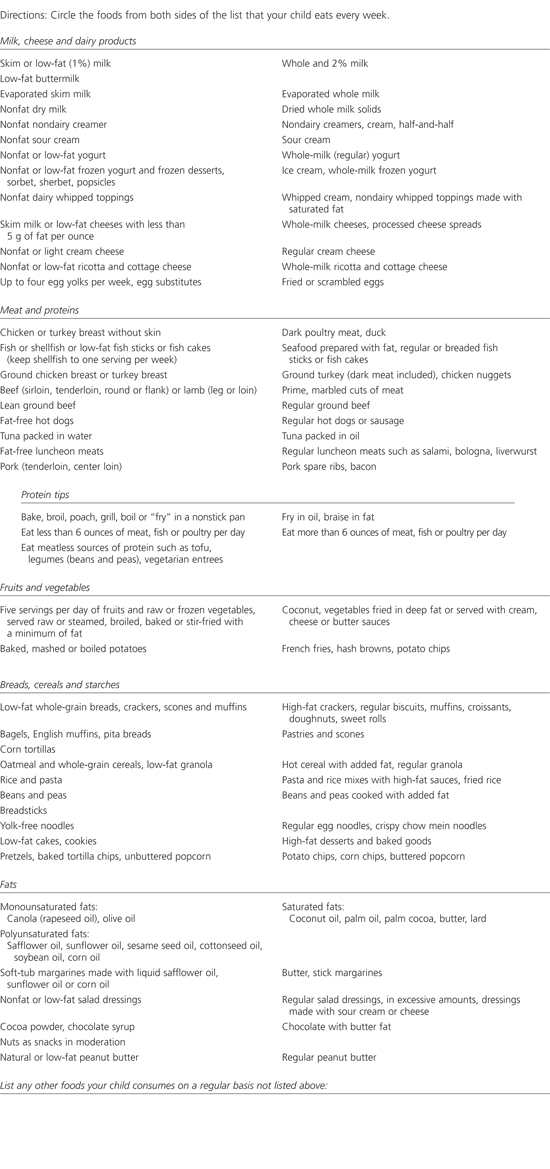
GOALS FOR DIETARY THERAPY
Although the ideal goal for dietary intervention is to lower the LDL cholesterol level to less than 110 mg per dL (2.85 mmol per L), in some cases this will not be possible. There are at least two reasons for this: (1) the child already consumes a heart-healthy diet or (2) the elevation may be high enough that the percentage of reduction achieved by dietary modification (typically no more than 20 percent and frequently less than 10 percent) still may not lower LDL cholesterol to the target goal. A more realistic goal for children and adolescents with a high level of LDL cholesterol is to reduce the level to lower than 130 mg per dL (3.35 mmol per L).
FOLLOW-UP AND INITIATION OF AHA STEP II DIET
To confirm the effectiveness of dietary changes, the LDL cholesterol level should be checked three to six months after treatment is started and yearly thereafter. Because lowering LDL cholesterol levels reduces the progression of CHD, any lowering of LDL cholesterol levels is valuable, even if the goal is not accomplished.
If the step I diet does not achieve the goal, the NCEP recommended dietary approach is for a trial of the more restrictive step II diet, which contains less than 7 percent of total calories from saturated fat and restricts dietary cholesterol to less than 75 mg per 1,000 kcal. Despite the seemingly modest reductions of the step II diet, there is the potential to develop nutritional deficiencies because of a more limited food selection. Therefore, patients placed on the step II diet should be closely monitored and should receive consultation with a clinician who has considerable nutritional expertise.
MONITORING FOR OTHER CARDIOVASCULAR RISK FACTORS
Other risk factors for cardiovascular disease, such as cigarette smoking, physical inactivity and obesity, are sometimes present in childhood and adolescence. Although they are not specifically addressed in this article, recommendations should be made for appropriate lifestyle modifications, such as maintaining a desirable weight, exercising, reducing sedentary activities (e.g., television watching) and smoking cessation. Involving all family members in the dietary changes and lifestyle modifications is important for long-term adherence to these treatment modalities.
Assessing Dietary Habits and Recommending Changes
Families need to learn to select foods that are low in total fat, particularly saturated fat, and cholesterol. The diet should include a variety of foods to ensure adequate intake of carbohydrates, protein and other essential nutrients, such as calcium and iron. Sufficient calories need to be consumed to ensure adequate growth and development.
In addition to lowering total fat and saturated fat intake, evidence has accumulated about the adverse effect of transunsaturated fatty acids (resulting from hydrogenation of oils, such as in margarines) on blood cholesterol levels.33 Hydrogenated fat consumption can be minimized by choosing liquid and soft fats over hardened fat products.
EVALUATION OF DIETARY HABITS
Ask the parents or child to keep a three-day food record before the next appointment (Figure 1). If they have not yet done this at the time of the follow-up visit, consider asking them to complete the food diary while they are in the waiting room, using a checklist of common foods. The items in the food diary can be arranged in two columns: foods listed on the left side are acceptable for eating on a daily basis; foods on the right side should be limited.
The physician can quickly scan the food diary and determine if the child is consuming too many inappropriate foods just by checking the right side of the page. Parent and child can be directed to use foods listed on the left side of the list as substitutes for those on the right side. They can also be referred to the AHA Cookbook for Kids. In addition, suggestions for lunchbox and snack ideas (see patient information handout) are helpful and should be provided. Families with Internet access should be encouraged to visit the AHA Web site (http://www.americanheart.org).
Table 2 provides the recommended daily caloric intake for girls and boys based on age and the 50th percentile of weight.34 Once the recommended total daily caloric intake is determined, as illustrated in Table 3, the maximal number of calories and grams that should come from fat and saturated fat can be calculated based on the NCEP recommendations for a step I diet (up to 30 percent of the daily caloric intake from all types of fat and up to 10 percent from saturated fat). Similar calculations can be made for the step II diet, using 7 percent of total daily calories as the upper limit for saturated fat. Total fat intake in children and adolescents should not be reduced to less than 20 percent of the daily caloric intake.32
| Total calories | Calories from fat | Grams from fat | ||
|---|---|---|---|---|
| Total fat (30% of calories) | Saturated fat (10% of calories) | Total fat (30% of calories) | Saturated fat (10% of calories) | |
| 2,000 | 600 calories | 200 calories | 67 g | 22 g |