
Am Fam Physician. 2000;61(6):1697-1704
See related patient information handouts on what you should know about herpes and what you can do about herpes, written by the author of this article.
Herpes simplex virus infection is increasingly common in the United States. New antiviral medications have expanded treatment options for the two most common cutaneous manifestations, orolabial and genital herpes. Acyclovir therapy remains an effective and often less expensive option. Famciclovir and valacyclovir offer improved oral bioavailability and convenient oral dosing schedules but are more expensive than acyclovir. Patients who have six or more recurrences of genital herpes per year can be treated with one of the following regimens: acyclovir, 400 mg twice daily; valacyclovir, 1 g daily; or famciclovir, 250 mg twice daily. These regimens are effective in suppressing 70 to 80 percent of symptomatic recurrences. Episodic treatment of recurrent genital herpes is of questionable benefit, but it may be helpful in appropriately selected patients. There is little evidence indicating benefit from treatment of recurrent orolabial herpes, which tends to be mild and infrequent.
Herpes simplex virus (HSV) affects more than one third of the world's population1 and is responsible for a wide array of human disease, with effects ranging from discomfort to death. Before the 1970s, when acyclovir (Zovirax) was introduced as an antiviral drug, cutaneous HSV infection was managed with drying agents and other local care. Newer antiviral drugs with once-daily dosage benefits have emerged during the past several years. Famciclovir (Famvir) and valacyclovir (Valtrex) offer effective and convenient therapeutic choices but are often more expensive than acyclovir.
The Virus and Pathogenesis
HSV is a double-stranded DNA virus that may enter the host through abraded skin or intact mucous membranes.1 Epithelial cells are the initial targets. Once infected, these cells die, releasing clear fluid intradermally to form vesicles and merging with other cells to create multinucleated giant cells.
Retrograde transport through adjacent neural tissue to sensory ganglia leads to lifelong latent infection.1 Reactivation of the virus is triggered by local or systemic stimuli such as immunodeficiency, trauma, fever, menstruation, ultraviolet (UV) light and sexual intercourse.1–3 Although emotional stress is assumed to trigger HSV recurrence, recent research fails to show a definite causative role.4 Once reactivated, the virus is transported by the neuron back to the epithelium, where more replication occurs, and another outbreak ensues.
HSV exists as two separate types, labeled 1 and 2, which have affinities for different body sites.2 Ninety percent of infections caused by HSV-2 are genital, and 90 percent of those caused by HSV-1 are oral; the reason for this division is unknown.5 In addition, oral HSV-1 infection recurs more frequently than oral HSV-2, and genital HSV-2 recurs more often than genital HSV-1.
Diagnosis
The diagnosis of genital HSV infection may be made clinically, but laboratory confirmation is recommended in patients presenting with primary or suspected recurrent infection. The gold standard of diagnosis is viral isolation by tissue culture,1 although this process can take as long as four to five days, and the sensitivity rate is only 70 to 80 percent. Despite these limitations, viral culture is still the diagnostic test of choice for HSV skin infections.
Tzanck preparations and antigen detection tests have lower sensitivity rates (50 to 70 percent) than viral culture.3 Serologic testing is extremely sensitive but is not helpful during primary infection because of the delay in antibody development.2 Polymerase chain reaction enzyme-linked immunosorbent assay (PCR-ELISA) is extremely sensitive (96 percent) and specific (99 percent) but expensive.1 For this reason, it is not used for the diagnosis of skin lesions but is the laboratory test of choice for diagnosing HSV encephalitis.
Antiviral Medications
Acyclovir, an acyclic guanosine analog, binds viral DNA polymerase, acting as a chain terminator and ending replication. Its mechanism of action necessitates early administration, because replication may end as soon as 48 hours into a recurrence.3
Oral bioavailability is only 15 to 30 percent; concentrations 10-fold higher can be achieved with intravenous administration.6 The half-life of acyclovir is about 2.5 hours, and the dosage must be adjusted in patients with renal failure. The drug penetrates well into most body tissues, including the brain, and crosses the placenta.
Acyclovir is a safe and extremely well-tolerated drug. Data from more than 35 million patients have been consistent and reassuring.6 Some authorities have proposed making acyclovir available as a nonprescription drug. Toxicity is rare, but in patients who are dehydrated or who have poor renal function, the drug can crystallize in the renal tubules, leading to a reversible creatinine elevation or, rarely, acute tubular necrosis. Adverse effects, usually mild, include nausea, vomiting, rash and headache. Lethargy, tremulousness, seizures and delirium have been reported rarely in studies of renally impaired patients.2,3
The Acyclovir in Pregnancy Registry has documented prenatal exposures in more than 850 women (with 578 first-trimester exposures) without any adverse outcomes.7 However, the total number of pregnancies monitored to-date may not be enough to detect defects that occur only infrequently.6 Therefore, the drug is labeled pregnancy category C by the U.S. Food and Drug Administration.
Famciclovir, another new antiviral medication, is the oral form of penciclovir, a purine analog similar to acyclovir. Oral bioavailability is 77 percent, and the drug is quickly converted to its active form.11 Mechanism and efficacy are similar to those of acyclovir.12 Famciclovir's intracellular half-life is 10 times longer than acyclovir's; despite this, dosing less frequently than twice daily is not recommended.13
Genital Herpes
Genital HSV infection is usually transmitted through sexual contact; therefore, it generally does not occur before adolescence. When genital herpes occurs in a preadolescent, the possibility of abuse must be considered, as with all sexually transmitted diseases in children.
Recent evidence indicates that 21.9 percent of all persons in the United States 12 years of age or older have serologic evidence of HSV-2 infection14; this figure has increased by 30 percent since the late 1970s. Independent risk factors include multiple sexual partners, increasing age, female gender, low socioeconomic status and human immunodeficiency virus (HIV) infection.1,2,14,15
CLINICAL PRESENTATION
Primary genital herpes has an incubation period of two to 12 days, with a mean of four days, followed by a prodrome of itching, burning or erythema.1 Multiple transient, painful vesicles then appear on the penis, perineum, vulva, vagina or cervix, and tender inguinal lymphadenopathy may follow3 (Figure 1). In immunocompetent patients, the initial ulceration crusts and heals by 14 to 21 days. Systemic symptoms are common in primary disease and include fever, headache, malaise, abdominal pain and myalgia.2,3 Recurrences are usually less severe and shorter in duration than the initial outbreak.1,3
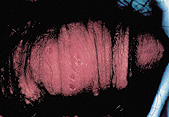
Shedding of viable viral particles happens frequently. Differences in shedding rates between genders have not been identified. Women with established genital HSV-2 infection have asymptomatic shedding 1 to 5 percent of days,1 and many patients, especially those with recent primary infection or coinfection with HIV, shed virus even more frequently.16,17 As many as 90 percent of those infected are unaware that they have herpes infection and may unknowingly shed virus and transmit infection.15
TREATMENT OF PRIMARY INFECTION
Antiviral therapy is recommended for an initial genital herpes outbreak, especially if the patient has systemic symptoms or is immunocompromised.2 Oral acyclovir is effective in reducing symptoms1 (Table 118 ). Intravenous administration may be required in immunocompromised patients and those with severe disseminated infection.1 Topical acyclovir reduces the duration of viral shedding and the length of time before all lesions become crusted, but this treatment is much less effective than oral or intravenous acyclovir.1
| Symptom/sign | Duration of symptoms/signs (days) | |
|---|---|---|
| Without acyclovir | With acyclovir* | |
| Viral shedding | 9 | 2 |
| Time until all lesions are crusted | 10 | 7 |
| Time until all lesions are healed | 16 | 10 |
| Local pain | 7 | 5 |
| Constitutional symptoms | 6 | 3 |
The oral acyclovir dosage for treatment of primary or initial nonprimary genital herpes is 200 mg five times daily for 10 days. This regimen does not influence the frequency or number of recurrences. It cannot eradicate latent virus and does not affect the long-term natural history of the infection.18,19
Few data are available regarding newer drugs in the treatment of primary disease. Valacyclovir, given twice daily, is indicated for the treatment of primary genital herpes but costs more than acyclovir. There is evidence that famciclovir, given three times daily, is as effective as acyclovir in the treatment of initial genital herpes infection,20 although it may be twice as expensive (Table 2).
TREATMENT OF RECURRENT INFECTION
Recurrences of herpes are often mild and infrequent, and most patients do not seek treatment. Drug therapy to prevent recurrences is available and effective, but because of cost and inconvenience issues, it is traditionally reserved for use in patients who have more than six outbreaks per year. Suppressive therapy in these patients is intended to reduce the frequency and severity of herpes symptoms, decrease the transmission of HSV to sexual partners and infants of infected mothers, and decrease the transmission of associated viral diseases (i.e., HIV). Unfortunately, only the first goal has yet proved to be attainable.
Acyclovir has been used to suppress recurrences of genital herpes, decreasing the frequency by as much as 80 percent and preventing recurrence by as much as 45 percent of patients.6 Successful suppression for as long as five years without adverse effects has been reported.3 The drug is titrated from a starting dose of 400 mg twice daily to achieve maximal efficacy with the lowest, least frequent dose.1 Therapy should be discontinued once a year to assess whether its continuation is necessary. In addition, cost and compliance should be discussed with the patient. Acyclovir resistance has not been a problem in immunocompetent patients but has been documented in 4 percent of HIV-infected persons.1
Large, randomized, double-blind trials have shown famciclovir and valacyclovir to be as effective as acyclovir in suppressing recurrent genital herpes10,12,13,21 (Table 3). Valacyclovir has the advantage of once-daily dosing.10 Famciclovir, despite its favorable intracellular pharmacokinetics, must be given twice daily to be effective.13
| Drug | Dosage | Decrease in recurrence rate (percentage) | Suggested for use in patients with ≥ 6 recurrences per year | Cost (generic)* |
|---|---|---|---|---|
| Acyclovir (Zovirax) | 400 mg twice daily | 78 to 79 | Yes | $5 (4) |
| Famciclovir (Famvir) | 250 mg twice daily | 79 | Yes | 7 |
| Valacyclovir (Valtrex) | 1 g once daily | 78 to 79 | Yes | 4 |
| 250 mg twice daily†‡ | 78 to 79 | Yes | 3 | |
| 500 mg once daily | 71 | No | 3 | |
| 250 mg once daily†‡ | 54 | No | 2 |
Aspirin, in a dosage of 125 mg daily, reduced active-infection days by nearly 50 percent in a small, unblinded pilot study.22 Future research should define whether this inexpensive alternative has a role in genital herpes suppression. Vaccines, which are still in the beginning stages of research, may in the future be capable of reducing the frequency and severity of recurrences.23
EPISODIC THERAPY
Episodic treatment is intended to diminish symptoms and infectivity during recurrences rather than reduce the frequency of recurrences. Acyclovir, taken within minutes to hours after the prodrome of recurrence begins, exerts a statistical, albeit minimal benefit in recurrent genital herpes infections.24 Famciclovir and valacyclovir have been shown to be slightly more effective for the treatment of recurrent infections.
Because the benefit, although statistically significant, is small, many have questioned the clinical usefulness of episodic treatment.25,26 Patients who have frequent recurrences overwhelmingly choose suppressive therapy rather than episodic therapy.13,27 In patients with fewer recurrences, however, it may be difficult to define what constitutes clinical significance in this stigmatizing, isolating illness. Because the number, severity and personal impact of recurrences vary so greatly, patients should be encouraged to participate in decisions regarding episodic antiviral therapy (Table 4).
| Drug | Dosage | Cost per episode* |
|---|---|---|
| Acyclovir (Zovirax) | 200 mg 5 times daily for 5 days | $32 (24–27) |
| 800 mg twice daily for 5 days | 49 (36–40) | |
| Famciclovir (Famvir) | 125 mg twice daily for 5 days | 28 |
| Valacyclovir (Valtrex) | 500 mg twice daily for 5 days | 30 |
PREVENTION
One hope in treating genital herpes is that the rate of shedding and transmission might be influenced, although a link between decreasing viral shedding and decreasing actual transmission has not yet been shown.28 Asymptomatic shedding decreases as much as 95 percent with suppressive acyclovir therapy,29,30 but some shedding still occurs. Potential preventive strategies are listed in Table 5.3,14,28,31,32
| Intervention | Effect |
|---|---|
| Abstention from sexual activity, limited lifetime number of sexual partners | Prevention of exposure and transmission of disease28,31 |
| Use of condoms | Protection against spread but not foolproof, because ulcers can occur on areas of the body not covered by condoms14 |
| Education about transmission and shedding | Avoidance of high-risk situations28,31 |
| Education about autoinoculation to other parts of the body (e.g., patting dry, not rubbing with towel) | Prevention of spread to nongenital areas of the body3 |
| Vaccination to prevent transmission | No effective vaccines currently available; mucosal immunization may be an emerging key32 |
Orolabial Herpes
Orolabial herpes (gingivostomatitis) is the most prevalent form of mucocutaneous herpes infection; 35 to 60 percent of white persons in the United States show serologic evidence of having been infected by HSV-1.1 Black persons and persons from low economic populations are infected earlier in life. Overall, the highest rate of infection occurs during the preschool years.1 Female gender, history of sexually transmitted diseases and multiple sexual partners have also been identified as risk factors for HSV-1 infection.33
CLINICAL PRESENTATION
Primary herpetic gingivostomatitis usually affects children under the age of five. It typically takes the form of painful vesicles and ulcerative erosions on the tongue, palate, gingiva, buccal mucosa and lips (Figure 2).
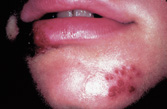
Edema, halitosis and drooling may be present, and tender submandibular or cervical lymphadenopathy is not uncommon.1 Hospitalization may be necessary when pain prevents eating or fluid intake.
Systemic symptoms are often present, including fever (38.4 to 40°C [101 to 104°F]), malaise and myalgia. The pharyngitis and flu-like symptoms are difficult to distinguish from mononucleosis in older patients. The duration of the illness is two to three weeks, and oral shedding of virus may continue for as long as 23 days.1
Recurrences typically occur two or three times a year. The duration is shorter and the discomfort less severe than in primary infections; the lesions are often single and more localized, and the vesicles heal completely by eight to 10 days. Pain diminishes quickly in four to five days.
UV radiation predictably triggers recurrence of orolabial HSV-1, an effect which, for unknown reasons, is not fully suppressed by acyclovir.34 Pharmacologic intervention is therefore more difficult in patients with orolabial infection.
TREATMENT OF PRIMARY INFECTION
Topical medication for HSV infection is generally not highly effective. Topical acyclovir has not proved to accelerate healing. Topical penciclovir, applied every two hours for four days, reduces clinical healing time by only about one day.1
In the treatment of primary orolabial herpes, oral acyclovir, in a dosage of 200 mg five times daily for five days, accelerates loss of crusts by one day (seven versus eight days) in adults1 and can reduce the mean duration of pain by 36 percent.35 The clinical importance of these effects is questionable. In one study involving children up to six years of age, acyclovir, in a dosage of 15 mg per kg five times daily for seven days, decreased the duration of oral lesions in primary infection from 10 days to four days, shortened viral shedding and reduced the duration of eating difficulties from seven days to four days.36 Standard analgesic therapy with acetaminophen or ibuprofen, careful monitoring of hydration status and aggressive early rehydration therapy are usually sufficient to avoid inpatient admission in most children.
Evidence from clinical trials of the newer antiviral agents as therapy for orolabial herpes is currently lacking.
TREATMENT OF RECURRENT INFECTION
Although long-term suppression of orolabial herpes has not been addressed by clinical trials, episodic prophylaxis has been studied because of the predictable trigger effect of UV radiation. A recent review37 found no evidence that topical acyclovir was helpful in preventing recurrence secondary to UV light exposure. Oral acyclovir, however, in dosages ranging from 400 to 1,000 mg daily, was effective in reducing by 50 to 78 percent the frequency of herpes labialis following UV light exposure. In another study involving skiers,38 oral acyclovir did not decrease the recurrence rate but lessened the severity of lesions when they occurred.
Short-term prophylactic therapy with acyclovir, therefore, may be desirable in some patients who anticipate intense exposure to UV light (e.g., skiers, or in those who work outdoors), although the clinical effect may vary.
Early treatment of recurrent orolabial HSV infection with high dosages of antiviral medication has recently been found to markedly decrease the size and duration of lesions. Famciclovir, in a dosage of 250 mg three times daily for five days, decreased mean lesion surface area by more than 50 percent and accelerated healing time from 5.8 days (placebo subjects) to 3.0 days (medicated group).39