
Am Fam Physician. 2000;61(8):2410-2416
This is part II of a two-part article on acute otitis media. Part I, “Improving Diagnostic Accuracy,“ appeared in the April 1 issue (Am Fam Physician 2000: 61:2051–6).
Antibiotic resistance is increasing among the pathogens that commonly cause acute otitis media. This development may merit changes in the traditional antibiotic treatment of acute otitis media. Risk factors for resistant pathogens include recent antibiotic treatment of acute otitis media, children in day care facilities, wintertime infections and acute otitis media in children less than two years of age. Amoxicillin remains the antibiotic of first choice, although a higher dosage (80 mg per kg per day) may be indicated to ensure eradication of resistant Streptococcus pneumoniae. Oral cefuroxime or amoxicillin-clavulanate and intramuscular ceftriaxone are suggested second-line choices for treatment failure. Compliance with antibiotic regimens is enhanced by selecting agents that require less frequent dosing (such as one or two times a day) and by prescribing shorter (five days or less) treatment courses. Selective use of tympanocentesis if the patient does not respond to empiric therapy can help confirm the diagnosis and guide effective therapy.
Differentiating among the many antibiotics that can be used to treat acute otitis media can be overwhelming (Table 1). In addition, the situation is becoming even more complex as a result of escalating antibiotic resistance among pathogens that cause acute otitis media.1–5 The pathogens and rates of resistance in acute otitis media have changed enough to make data obtained before 19936 not reflective of the current situation. The primary explanation for the increasing rates of antibiotic resistance is repeated exposure of these bacteria to antibiotics and geographic spread of resistant strains.7
| Penicillins |
| Amoxicillin |
| Amoxicillin-clavulanate (Augmentin) |
| Sulfa-based combinations |
| Erythromycin-sulfisoxazole (Pediazole) |
| Trimethoprim-sulfamethoxazole (Bactrim, Septra) |
| Macrolide/azalide |
| Azithromycin (Zithromax) |
| Clarithromycin (Biaxin) |
| Second-generation cephalosporins |
| Cefaclor (Ceclor) |
| Cefprozil (Cefzil) |
| Cefuroxime axetil (Ceftin) |
| Loracarbef (Lorabid) |
| Third-generation cephalosporins |
| Cefdinir (Omnicef) |
| Cefixime (Suprax) |
| Cefpodoxime proxetil (Vantin) |
| Ceftibuten (Cedax) |
| Ceftriaxone (Rocephin) |
The percentage of Streptococcus pneumoniae strains found to demonstrate a reduced susceptibility to penicillin (and amoxicillin) now ranges from 30 to 60 percent in the United States.2,4,5 Resistance of these organisms to trimethoprim-sulfamethoxazole (Bactrim, Septra) generally exceeds 50 percent.5 Quinolone resistance among S. pneumoniae strains is also rising.8 The proportion of beta-lactamase–producing Haemophilus influenzae and Morexalla catarrhalis strains in acute otitis media has nearly tripled in the past decade, from rates of 15 to 20 percent in the 1980s to currently reported rates of up to 55 percent for H. influenzae1–3,9 and virtually 100 percent for M. catarrhalis. Similarly, resistance of H. influenzae to trimethoprim-sulfamethoxazole has increased.10,11
Antibiotic resistance occurs most frequently in patients who were recently treated for acute otitis media. Our group found a 46 percent rate of penicillin-resistant S. pneumoniae in patients recently treated for acute otitis media,9 and 33 percent of these strains were highly resistant.9 The incidence of resistant pathogens is higher in children who attend day care facilities, in the wintertime12 and in children younger than two years of age.13
Much misinformation has been generated regarding the role of Mycoplasma pneumoniae, Chlamydia pneumoniae and viruses in acute otitis media. In a study conducted in the 1970s, M. pneumoniae was recovered from only one of 771 tympanocentesis samples.14 In a 1997 study, C. pneumoniae was isolated from eight of 101 children with acute otitis media; however, it was the sole microbe isolated in only two children.15 While viral upper respiratory infections frequently precede acute otitis media, and while viruses are not uncommonly isolated along with bacterial pathogens from middle ear effusions, viruses have been found to be the sole pathogen in fewer than 10 percent of cases of acute otitis media.16 Anaerobes may play a role in chronic otitis media but not in acute otitis media.
Impact of Antibiotic Therapy on Outcome
In the past, high cure rates were reported for most antibiotics used in the treatment of acute otitis media. Ten years ago, S. pneumoniae was almost universally susceptible to amoxicillin, and 20 years ago, H. influenzae infrequently produced the beta-lactamase enzyme, and M. catarrhalis was not pathogenic. Clinical trials of antibiotics almost always revealed successful clinical outcomes. However, these high cure rates were often unreliable because of a number of design flaws. Explanations for such overly positive results included the following: (1) overdiagnosis of acute otitis media at study entry, (2) inclusion of patients with only mild to moderate acute otitis media, (3) exclusion of difficult-to-treat cases and (4) use of overly broad criteria (symptom resolution only) for the definition of clinical “cure.”
Also, acute otitis media has a favorable natural history regardless of antibiotic use. A meta-analysis of studies conducted from 1966 to 199217 concluded that the overall rate of spontaneous resolution of acute otitis media was 81 percent. The data revealed that the benefit of antibiotics in acute otitis media was 13.7 percent over placebo. Examined another way in another study,18 antibiotics were assessed to offer resolution of pain approximately two days sooner than when no antibiotic therapy was given or when treatment consisted of analgesics alone.
The “marginal” benefits of antibiotic therapy have led some authorities to propose non-treatment paradigms for acute otitis media19 or to advocate a two-day delay in treatment to see if symptoms resolve on their own. While such recommendations may appeal to those with a predisposition to withhold antibiotics in cases of uncomplicated acute otitis media, they may be flawed because the data on which they are based come from older studies in which bacterial resistance was not as prevalent as it is currently.
Bacterial Resistance After Antibiotic Therapy Failures
During the 1980s, H. influenzae was the most common (46 to 62 percent) pathogen isolated in patients who failed to respond to initial treatment of acute otitis media.20 Currently, it appears that S. pneumoniae, particularly penicillin-resistant strains, accounts for an increasing number of pathogens in patients who fail to respond to antibiotic therapy.9,21,22 The highest risk for penicillin-resistant S. pneumoniae occurs in patients who have been treated recently with antibiotics (particularly within the preceding month) or who are not improving during a course of antibiotic therapy.9,21,22
With persistent and recurrent acute otitis media, treatment success rates can be expected to be in the range of 60 to 70 percent, even when the most efficacious broad-spectrum oral antibiotics are chosen.1,21 Currently, minimal data are available regarding the treatment of acute otitis media caused by highly penicillin-resistant S. pneumoniae. Thus far, only five antibiotics—high-dose amoxicillin (80 mg per kg per day), amoxicillin-clavulanate (Augmentin), cefuroxime (Ceftin), cefprozil (Cefzil) and ceftriaxone (Rocephin)—have demonstrated a modest degree (60 to 80 percent) of clinical efficacy in the treatment of acute otitis media caused by penicillin-resistant S. pneumoniae.9,22–24 Studies of other broad-spectrum agents are currently under way.
CDC Recommendations for Management of Acute Otitis Media
The Centers for Disease Control and Prevention (CDC) report from the drug-resistant S. pneumoniae therapeutic working group, which included representatives of the American Academy of Family Physicians, gave specific recommendations for the treatment of acute otitis media (Table 2).25 The CDC working group distinguished initial treatment options on the basis of whether or not the patient had recently received antibiotic therapy, because recent exposure clearly increases the risk of resistant pathogens.
A switch in empiric therapy is recommended on day 3 of therapy and/or 10 to 28 days after initial diagnosis in cases of clinically defined treatment failure. According to the CDC report, agents selected for alternative therapy meet two criteria: (1) the antibiotics are effective against S. pneumoniae, including most drug-resistant strains, and (2) the antibiotics are effective against H. influenzae and M. catarrhalis, including beta-lactamase–resistant strains. For empiric therapy after amoxicillin treatment failure, three agents were selected: high-dose amoxicillin-clavulanate, cefuroxime and intramuscular ceftriaxone. With currently available U.S. formulations, the amoxicillin-clavulanate regimen would require two prescriptions: one for amoxicillin (40 mg per kg per day) and one for amoxicillin-clavulanate (also at 40 mg per kg per day of amoxicillin). In contrast to the use of ceftriaxone in uncomplicated acute otitis media, where a single injection is acceptable, treatment with ceftriaxone when resistant bacteria are suspected requires two to three injections over two to three days. Two other antibiotics—cefprozil and cefpodoxime (Vantin)—were strongly considered as empiric candidates but were not included among the preferred choices because more data are needed. Cefdinir (Omnicef), the newest antibiotic for acute otitis media, was not labeled at the time of the CDC review.
Tympanocentesis was noted as an option in cases of treatment failure (day 3 of antibiotic therapy and days 10 to 28 after completion of treatment). Tympanocentesis was viewed as “particularly important if a child has recently received several courses of antimicrobial therapy and is therefore more likely to harbor a multiply-resistant strain.”25 In this context, the working group stated, “In an era of increasing antimicrobial resistance, clinicians treating children with acute otitis media should consider developing the capacity to perform tympanocentesis themselves or establish ready referral mechanisms to a clinician with this capacity.” If tympanocentesis is performed and S. pneumoniae is isolated, clindamycin then becomes a treatment option.
INTERPRETING THE RECOMMENDATIONS OF THE CDC WORKING GROUP
According to the CDC report, amoxicillin remains the initial drug of choice for the treatment of acute otitis media. Higher dosages of amoxicillin (80 mg per kg per day rather than the usual 40 mg per kg per day) are recommended to address the issue of penicillin-resistant pneumococci.25 The increased dosage produces higher levels of amoxicillin in middle ear fluid.26 However, regardless of the dosage, amoxicillin will not eradicate beta-lactamase–producing H. influenzae or M. catarrhalis. For this reason, alternatives to amoxicillin should ideally be effective against these beta-lactamase–producing pathogens.
The “pharmacodynamics model”27,28 heavily influenced the CDC recommendations. In this model, clinical cure is thought to correlate with demonstrated penetrance of the antibiotic in the middle ear at a level known to be high enough to kill bacterial pathogens that cause acute otitis media. Nevertheless, this model has three shortcomings: (1) While bacteriologic eradication correlates with a successful clinical outcome, clinical success occurs in more than 60 percent of patients even when bacteriologic eradication is not achieved. Eventually almost everyone gets better. (2) Validation of the pharmacodynamic model relies on tympanocentesis for identification of the causative bacteria and measurement of antibiotic levels in middle ear fluid. Some antibiotics, such as azithromycin (Zithromax) and clarithromycin (Biaxin), concentrate intracellularly, not in middle ear fluid, and are bacteriostatic, not bactericidal. A model looking for certain drug levels and bacterial eradication may not accurately assess the efficacy of such agents. (3) The drug levels for bacterial killing used by the CDC were based on standards that changed six months after the CDC publication.
The CDC recommendations include the possibility of performing tympanocentesis in selected cases to guide management of refractory acute otitis media. Few family physicians, however, perform this procedure. Otolaryngologists are infrequently available to accommodate a same-day referral for the procedure, and still fewer have done it without the benefit of general anesthesia.
Empiric Antibiotic Selection Without Tympanocentesis
The major considerations in empiric antibiotic selection for acute otitis media include comparative drug efficacy, safety, compliance potential and cost.1 Sources of information on antibiotics are often confusing and conflicting. Pharmaceutical representatives produce brochures touting their drug as effective and perhaps better in some other way (for example, a low diarrhea rate, palatable taste, low dosing frequency, no need for refrigeration or a few dollars lower in cost).
There is little to distinguish one antibiotic from another in terms of safety profiles. All of the antibiotics used for acute otitis media are generally quite safe. Compliance, duration of therapy and cost are important issues. The main determinants of compliance appear to be frequency of dosing, palatability of the agent and duration of therapy. Less frequent doses (once or twice a day) are more desirable than more frequent doses, which interfere with daily routines. In many instances, palatability ultimately determines compliance in children.
Patients prefer a shorter course of therapy (five days or less) rather than the traditional 10- to 14-day courses often used in the United States. One recent survey verified that many patients and parents only continue antibiotic therapy until symptoms resolve, perhaps followed by an additional one or two days.29 The remainder of the prescription is usually saved for future use when similar symptoms arise.29
Antibiotic cost is an interesting component of the treatment paradigm. Drug costs alone rarely reflect the total cost of treating an illness. For example, three office visits and three injections of intramuscular ceftriaxone would seem to greatly escalate the cost of treating acute otitis media. However, the costs of loss of work or school attendance as a result of treatment failure and of repeat office visits for additional evaluation are also important factors, but they are often overlooked when the comparative costs of treatment include only the cost of the antibiotic.
Pathogen-Directed Antibiotic Selection
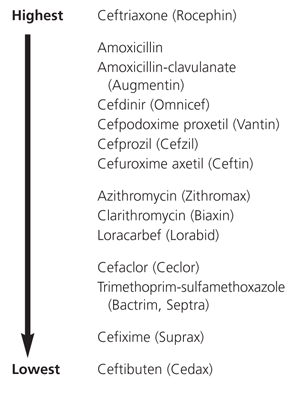
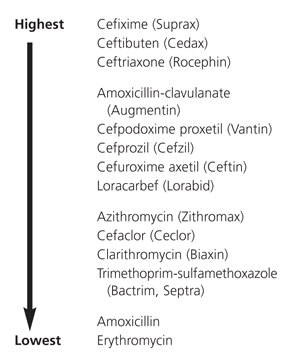
Tympanocentesis allows isolation of the bacterial pathogen from middle ear fluid in approximately two thirds of children with acute otitis media30 and in 50 percent of children with persistent or recurrent otitis media.21 Even though the otoscopic examination may show tympanic membrane inflammation, the middle ear fluid may be sterile by the time the patient seeks care. When a tympanocentesis is performed, management options include (1) waiting for culture results before an antibiotic is selected or (2) providing the patient with two days of empiric antibiotic treatment, then changing the prescription or discontinuing treatment, depending on the culture results. If bacteria are not isolated in the specimen, no antibiotic therapy is required.
Despite the cost of tympanocentesis, culture and susceptibility testing, the specific information obtained can provide invaluable guidance in the selection of antibiotics for the management of difficult cases not responding to empiric treatment.
Final Comment
Many children with acute otitis media do not benefit from antimicrobial therapy because the etiology of their illness is not bacterial or the infection is cleared by the immune system without the use of a drug. At present, we do not have clinical criteria for distinguishing which children are in need of antibiotic therapy for acute otitis media.
Educational programs for patients as well as physicians are needed to discourage inappropriate antibiotic use. Tympanocentesis in selected cases of refractory or recurrent acute otitis media permits the use of pathogen-directed antibiotic therapy. Even with our best efforts, antimicrobial resistance is likely to continue to escalate, calling for the development of effective new antibiotics to treat these infections. Clinical trials are now ongoing with new families of drugs for acute otitis media, including new oral quinolones, oxazolidinones, streptogramins and ketolides. Also, conjugate pneumococcal vaccines are pending licensure and will hopefully prove efficacious in reducing cases of acute otitis media caused by S. pneumoniae.