
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2000;61(12):3601-3607
See related patient information handout on uterine fibroid embolization, written by the author of this article.
Interventional radiologists have performed uterine artery embolization to treat women with emergency uterine bleeding since the 1970s. In this procedure, the physician guides a small angiographic catheter into the uterine arteries and injects a stream of tiny particles that decreases blood flow to the uterus. It is now considered a safe and highly effective nonsurgical treatment of women with symptomatic uterine fibroid tumors. Uterine fibroid embolization has several advantages over conventional hormonal suppression and surgical procedures, including avoidance of the side effects of drug therapy and the physical and psychologic trauma of surgery. In addition, after uterine fibroid embolization, patients can normally resume their usual activities several weeks earlier than they can after hysterectomy. Along with hysteroscopic resection, myolysis and laparoscopic myomectomy, uterine fibroid embolization widens treatment options for patients who desire to avoid hysterectomy.
Uterine fibroids affect millions of women and may account for 60 percent of the 600,000 hysterectomies performed in the United States each year.1 Uterine fibroids may be asymptomatic but often cause menorraghia, dyspareunia and “bulk symptoms” (e.g., low back pain, urinary frequency and constipation). Uterine fibroids have also been associated with infertility. As an alternative to hysterectomy, uterine fibroid embolization (UFE) avoids the complications and side effects associated with hysterectomy, which include a six-week recovery period, a 2 percent risk of postoperative bleeding and a 15 to 38 percent risk of a postoperative febrile illness.2 A decrease in sexual function, depression and an increased incidence of cardiovascular disease have also been reported following hysterectomy.2
Family physicians commonly encounter patients with symptomatic uterine fibroids and, because more patients desire uterine-conserving treatments, family physicians should be familiar with them. UFE, a relatively new, nonsurgical treatment for women with uterine fibroids, is performed by interventional radiologists. Gynecologists are performing a variety of other uterine-sparing treatments in women with symptomatic uterine fibroids, including myomectomy, myolysis hysteroscopic resection and endometrial ablation. A detailed discussion of these procedures is beyond the scope of this article; however, Table 13–14 compares UFE with other uterine-sparing therapies.
| Treatment | Description | Advantages | Disadvantages |
|---|---|---|---|
| GnRH therapy | Medical therapy to suppress estrogen production and shrink uterine fibroids | Shrinkage of uterine fibroids may allow removal with less blood loss, or removal by laparoscope or hysteroscope | Induces premature menopause-like symptoms; associated with premature bone mineral loss3; rapid regrowth of fibroids when therapy is discontinued |
| Abdominal myomectomy | Open abdominal surgery to resect symptomatic uterine fibroids | Allows uterine conservation, usually in women who desire fertility | Postoperative recovery period of six weeks; general anesthesia required; transfusion rate of 3 to 20 percent3,4; adhesions may cause problems5; recurrence rate of 10 to 27 percent5,6 |
| Laparoscopic myomectomy | Laparoscopic removal of uterine fibroids | Much shorter recovery period than with abdominal myomectomy; best suited for pedunculated and subserosal fibroids or smaller intramural fibroids7,8 | Large, multiple or deep uterine fibroids are problematic; procedure-related adhesion formation may be significant; general anesthesia required7,8 |
| Laparoscopic myolysis | Laser probe used to heat coagulate uterine fibroids | Treated uterine fibroids may shrink up to 40 percent by 6-month follow-up7 | “Dense and fibrous adhesions” noted at second-look laparoscopy7* |
| Hysteroscopic resection and/or endometrial ablation | Hysteroscope is inserted into endometrial cavity, guiding the resection of submucous fibroids; endometrium is scraped and burned to create amenorrhea | Outpatient procedure for bleeding patients; short recovery period | Mortality from fluid overload and infection reported but rare; a 32 percent failure rate reported at 2 years post-ablation9 with a high rate (52 percent) of adenomyosis, possibly caused by ablation10; destroys fertility potential; a 13 percent rate of synechiae formation following hysteroscopic resection without ablation10 |
| Uterine fibroid embolization | An arteriographic catheter is passed through the femoral artery into the uterine arteries; tiny particles are injected that block blood flow inside the fibroids and cause infarct; other uterine structures are spared; uterine fibroids shrink, relieving symptoms | Abnormal bleeding and “bulk symptoms” improved in about 80 to 90 percent of patients11,12; surgical incision and general anesthesia not required; no blood loss; all fibroids treated at once13; no recurrences noted14 †; return to normal activities in 7 to 10 days | As expensive as hysterectomy; effect on fertility uncertain; delayed infection may occur in a small percentage of patients; availability to all patients may be limited‡; long-term follow-up data unavailable; some HMOs may not cover procedure cost |
Patient Selection for UFE
[ corrected] The ideal candidate for UFE is a postfertility, premenopausal patient with symptomatic uterine fibroids who strongly desires to avoid hysterectomy. Although there is no fixed size limitation, patients with pedunculated subserosal fibroids are not considered ideal candidates. UFE may be a particularly attractive option for patients who wish to avoid the possibility of blood transfusions for health or religious reasons. Patients with a contraindication to general anesthesia might also benefit from UFE.
At this time, UFE is not commonly being performed on women who desire future fertility because its effects on fertility are unknown. In these patients, myomectomy is the traditional treatment, although adhesions following myomectomy may obstruct fertility. Depending on the size and number of uterine fibroids, a myomectomy procedure may not be indicated in some patients. In these patients, uterine fibroid embolization will allow uterine preservation.
The Procedure
In performing therapeutic embolization, the interventional radiologist can selectively block blood flow to abnormal bleeding or tumor blood vessels anywhere in the body by injecting various embolic agents through angiographic catheters. The catheter is guided to the target under fluoroscopy. Since the 1970s, uterine artery embolization has been successfully performed to stop postpartum hemorrhage.15
A Parisian gynecologist noted shrinkage of uterine fibroids in patients who had undergone preoperative or emergency embolization. In 1995, he published the first report of uterine artery embolization as the primary treatment of women with uterine fibroids.16 Shortly thereafter, interventional radiologists in the United States began performing embolization on women with uterine fibroids.17 More than 5,000 UFEs have now been performed in the United States.
PREOPERATIVE EVALUATION
Before performing UFE, a thorough gynecologic evaluation is needed to determine that uterine fibroids are the direct cause of the patient's symptoms. After obtaining a patient history and conducting a physical examination, the patient should undergo magnetic resonance imaging (MRI) or ultrasonography of the uterus and ovaries. An MRI can more reliably differentiate uterine from ovarian masses. Patients with suspected endometriosis may require laparoscopy, and most patients with menorraghia should undergo an endometrial biopsy to exclude endometrial carcinoma. UFE is contraindicated in patients with active pelvic infection, renal insufficiency or a severe allergy to iodinated contrast material.
UFE TECHNIQUE
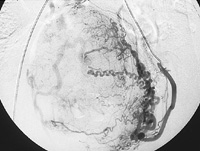
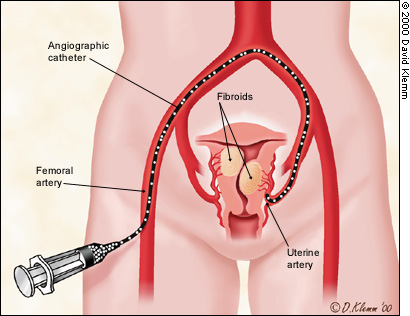
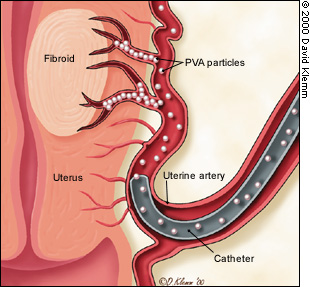
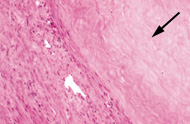
Before the procedure, intravenous antibiotics are administered. With the patient under mild intravenous sedation and local anesthesia, a small angiographic catheter is introduced into the femoral artery and guided into the left uterine artery. Arteriography is performed (Figure 1), and tiny particles of polyvinyl alcohol (PVA), approximately 500 μm in diameter, are injected in a slurry into the artery (Figure 2a). The process is repeated in the right uterine artery. (These particles have been used for embolization in many parts of the human body for more than 20 years without any significant reaction attributed to the agent.18) PVA flows into the hypervascular uterine fibroids (Figure 2b), blocking small arteries and causing ischemic necrosis. Normal myometrium is unharmed because it is supplied by multiple collateral arteries. A pathology section showing the effect of embolization on a fibroid and on normal myometrium is shown in Figure 3. After the right and left uterine arteries are embolized, the catheter is removed, and the patient undergoes standard postarteriographic monitoring and recovery.
Results
SHORT-TERM RESULTS
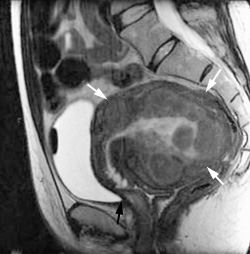
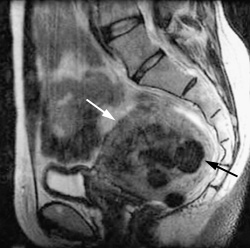
Infarcted uterine fibroids undergo variable shrinkage that averages 48 to 78 percent in volume.11 Total uterine volume decreases an average of 50 percent.11,12 Figure 4a depicts a pre-UFE uterus containing multiple uterine fibroids. At six-month follow-up, the size of the uterus has decreased by 50 percent (Figure 4b). From 81 to 92 percent of patients with fibroid-related menorrhagia who are treated with UFE report a significant improvement or resolution of abnormal uterine bleeding, while 79 to 92 percent of patients with bulk symptoms report significant improvement.11,12 Resumption of normal activities within four days was reported in 80 percent of patients, and 90 percent of patients reported a resumption of normal activities within 10 days.12,19 In one series12 of 305 patients treated with UFE, 86 percent indicated satisfaction with the results of the procedure.
Although a few patients who have undergone UFE have become pregnant and experienced successful deliveries,3 the effect of UFE on fertility has not been studied in a randomized fashion. One group of 27 women who underwent UFE for emergency hemorrhage reported no adverse effects on menses or fertility on follow-up evaluation.20 However, premature menopause induced by the procedure has been reported.
LONG-TERM RESULTS
The long-term effectiveness of UFE is unknown at this time. It is known that patients who have undergone a surgical myomectomy for uterine fibroids have a 10 to 27 percent recurrence rate.5,6 Recently, the results of a study were presented involving 184 patients who had undegone UFE over a six-year period. No recurrences were noted in this group of patients.14 More follow-up is needed to determine if UFE will prove to be more durable than myomectomy or other uterine-conserving surgical procedures.
Side Effects and Complications
After the procedure, almost all patients experience six to 12 hours of variably intense, cramping pelvic pain that may be treated with oral or intravenous analgesia. Epidural analgesia may be used in patients who experience severe pain. The pain diminishes during the first week following UFE, and patients can be maintained on oral analgesics. Approximately 15 to 30 percent of patients may experience a variable “postembolization syndrome,” with fever and malaise occuring during the first post-procedure week, but this syndrome resolves spontaneously and may be related to the release of tissue-breakdown products from degenerating uterine fibroids.17
Occasionally, submucosal fibroids become necrotic and may be expelled from the uterus following UFE. Reports of serious infection have been uncommon. A review of four series with a total of 751 patients reveals that 0.7 percent of patients had infection or severe ischemia requiring hysterectomy.3,11,12,21 Less severe infections may occur more commonly and may be managed without hysterectomy.21 One death from sepsis has been reported in the literature out of approximately 6,500 procedures performed worldwide.22
A few cases of temporary amenorrhea and post-procedure menopause have occurred and, although the incidence of these conditions is low, they must be considered a possible risk.3,19 Radiation exposure to the ovaries may be as high as 20 cGy with the use of continuous fluoroscopy.23 This amount is equivalent to approximately five computed tomographic (CT) scans of the pelvis. Using modern intermittent fluoroscopy, however, much lower doses would be expected, and much larger absorbed radiation doses have not caused an increase of genetic defects in offspring.23 One possible serious complication is “misembolization” which can damage other pelvic organs. This complication has not yet been reported in association with UFEs performed in the United States, and bleeding episodes that require transfusion are rare.
Advantages of UFE
UFE has several potential advantages over hysterectomy, myomectomy and hormonal suppression. Unlike myomectomy or hysterectomy, UFE involves virtually no blood loss or risk of blood transfusion.2,4,5 General anesthesia and surgical incisions are avoided. Recovery is weeks shorter than recovery from hysterectomy or open myomectomy (seven to 10 days versus six weeks),2,5,19 and early menopause-like symptoms are rarely induced as a result of UFE, as are often seen with gonadotropin-releasing hormone (GnRh) therapy. All fibroids are treated at once, which is not the case with myomectomy.13 UFE recurrence rates appear to be lower than those of myomectomy.14
Availability
In a selected patient population, UFE is a safe and highly effective treatment of women with symptomatic uterine fibroids. In the future, indications for its use may expand to include women who desire future fertility or even women who have fibroid-associated infertility. Although costs may vary significantly among institutions, it is likely that the cost of this procedure will approximate the cost of an abdominal hysterectomy.
One of the disadvantages associated with UFE is that it is currently somewhat difficult for women to learn about the procedure or its accessibility in their area. Some gynecologists may be unfamiliar with it or may counsel patients to stay with the tried and true surgical procedures. At this time, UFE is being performed widely in the United States and Canada. A directory of physicians who are performing this procedure in North America is now available through the Society of Cardiovascular & Interventional Radiology (10201 Lee Highway, Suite 500, Fairfax, VA 22030; telephone, 800-488-7284; Web site, http://www.scvir.org/).