
Am Fam Physician. 2000;62(2):401-404
Pulmonary edema is a potentially life-threatening complication of acute airway obstruction. It develops rapidly, without warning, in persons who are otherwise well. Two forms of postobstructive pulmonary edema (POPE) have been identified. POPE I follows sudden, severe upper airway obstruction. POPE II occurs following surgical relief of chronic upper airway obstruction. Treatment for both is supportive. Full and rapid recovery can be expected with appropriate management.
Family physicians must be able to recognize and initiate treatment for conditions that are uncommon but life threatening. Postobstructive pulmonary edema (POPE) is one of these conditions. Patients with POPE develop sudden, unexpected and often severe pulmonary edema. POPE follows an episode of acute airway obstruction or the relief of chronic upper airway obstruction1 in patients otherwise not at risk for pulmonary edema. Family physicians care for patients at risk of POPE in their offices, emergency departments, critical care units, medical wards and recovery rooms. Awareness of this uncommon condition is crucial if the family physician is to make an early diagnosis and initiate successful treatment.
Illustrative Case
A 12-year-old girl underwent an uncomplicated tonsillectomy and adenoidectomy under general endotracheal anesthesia. Both tonsils were markedly enlarged.
Immediately on arrival to the postanesthesia care unit, she became restless with signs of respiratory distress. Stridor was absent, and she had no signs of airway obstruction. Pulse oximetry was unobtainable because of patient movement. Reintubation was accomplished with difficulty because of copious quantities of blood-tinged secretions in the oral cavity. At the time of intubation, there was no evidence of laryngospasm and no gastric contents were noted.
Mechanical ventilation with 100 percent oxygen resulted in an oxygen saturation of 88 percent. Furosemide and morphine sulfate were administered intravenously with a brisk diuresis of 1,000 mL. Oxygen saturation slowly increased to 95 percent. A portable chest radiograph (Figure 1) demonstrated pulmonary edema. The patient was transferred to a tertiary care center that had a pediatric intensive care unit; she recovered fully and was discharged several days later.
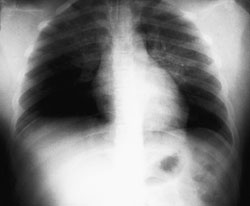
Background
In 1927, studies of dogs demonstrated that acute airway obstruction produced pulmonary edema.2,3 POPE was first described in humans in 1973.4 In 1977, two children were reported5 to have pulmonary edema during episodes of croup and epiglottitis. Also in 1977, a report was published6,7 of three adult patients who experienced the onset of pulmonary edema minutes to hours after severe, acute upper airway obstruction. Several case series have subsequently been reported.
Clinical Features
POPE is the sudden onset of pulmonary edema following upper airway obstruction. There are two recognized types of POPE.1 Type I follows a sudden, severe episode of upper airway obstruction such as postextubation laryngospasm,8 epiglottitis, croup,5,9,10 and choking,11,12 and is seen in strangulation and hanging.6 Type I POPE may be associated with any cause of acute airway obstruction. Type II POPE develops after surgical relief of chronic upper airway obstruction. Reported causes include tonsillectomy1 and removal of upper airway tumors.13 The illustrative case described above is typical of type II POPE.
Type I and type II POPE present with acute respiratory distress (Table 1). Clinical signs include tachypnea, tachycardia, rales and ronchi. Type I POPE usually occurs within 60 minutes of a precipitating event,14 but the onset has been delayed for up to six hours in some case reports.3,7,10,15,16 Type II POPE develops soon after relief of chronic upper airway obstruction.11,13
| Type I POPE |
| Postextubation laryngospasm |
| Epiglottitis |
| Croup |
| Choking/foreign body |
| Strangulation |
| Hanging |
| Endotracheal tube obstruction |
| Laryngeal tumor |
| Goiter |
| Mononucleosis |
| Postoperative vocal cord paralysis |
| Migration of Foley catheter balloon |
| used to tamponade epistaxis |
| Near drowning |
| Intraoperative direct suctioning |
| of endotracheal tube adapter |
| Type II POPE |
| Post-tonsillectomy/adenoidectomy |
| Post-removal of upper airway tumor |
| Choanal stenosis |
| Hypertrophic redundant uvula |
Incidence
The incidence of POPE is difficult to ascertain. Most reports are of single cases or case series. One study,9 however, reports a series of 176 children with severe upper airway obstruction who were treated in two children's hospital intensive care units. In this study, 167 children experienced acute upper airway obstruction and nine had chronic obstruction. Twenty children developed pulmonary edema. Sixteen of the 20 cases followed acute airway obstruction from croup, epiglottitis or postextubation subglottic edema (POPE I) for an incidence of 16/167 or 9.6 percent. Four of the 20 cases occurred in nine patients who underwent surgical relief of chronic upper airway obstruction (POPE II) for a remarkable incidence of 4/9 or 44 percent.9
In one study,17 a series of 53 consecutive pediatric patients undergoing adenotonsillectomy at a tertiary university hospital showed intraoperative or postoperative pulmonary edema in five children, for an incidence of 9.4 percent. POPE can present as a radiologic finding alone, suggesting that many cases may not be identified clinically. In a series10 of chest radiographs in 21 children intubated for acute upper airway obstruction, six (29 percent) developed radiographic evidence of POPE. While these series represent experiences in a tertiary care setting, the surprising frequency of POPE suggests that it is not rare.
Etiology
The pathogenesis of POPE I is multifactorial.3,18,19 Forceful attempts to inhale against an obstruction create highly negative intrathoracic pressure, which causes increased venous return, decreased cardiac output and fluid transudation into the alveolar space. The importance of vigorous inspiratory effort in POPE I is supported by the apparent increase in susceptibility to this condition in young athletic men20–23 who, because of their chest wall musculature, are able to generate extremely high negative inspiratory pressures. Other factors may also contribute, including direct suctioning of endotracheal tube adaptors during thoracotomy24; narcotic use; short neck; obesity25; obstructive apnea12; nasal, oral or pharyngeal surgery or pathology14; vocal cord paralysis26; conditions leading to increased capillary-alveolar pressure gradients27; endotracheal tube obstruction28; and premature extubation.29
The cause of POPE II is less clear than that of POPE I. It appears that the obstructing lesion produces a modest level of positive end-expiratory pressure (PEEP) and increases end-expiratory lung volume. Relief of the obstruction removes the PEEP and returns lung volumes and pressures to normal. It is postulated that altered permeability and previously occult interstitial fluid do not resolve immediately. The sudden removal of the PEEP leads to interstitial fluid transudation and pulmonary edema.1,3 POPE II is much less commonly reported than POPE I and predictive factors have not been clearly elucidated.
Diagnosis
POPE requires rapid intervention and may be confused with other causes of postoperative respiratory distress. Although symptoms usually develop within one hour of the precipitating event, delayed onsets have been reported.5,7,13,15,25,30 The presence of agitation, tachypnea, tachycardia, frothy pink pulmonary secretions, rales and progressive oxygen desaturation suggests the diagnosis of POPE in the appropriate setting. Chest radiograph findings of pulmonary edema support the diagnosis. Other causes of pulmonary edema should be considered (Table 2). The absence of gastric contents in pulmonary secretions, a history of normal cardiac function and, particularly, the occurrence of such symptoms in a vigorous young person makes the diagnosis of POPE more likely.
Prognosis and Treatment
Most cases of POPE respond promptly to appropriate treatment. One reported case31 of POPE I, however, in an otherwise healthy 43-year-old man with epiglottitis, progressed to adult respiratory distress syndrome and death. Treatment consists of supplemental oxygen and support. Intubation and the application of
low levels of PEEP (5 cm H2O) have been employed in most reported cases. It is not clear, however, if PEEP is required and its use must be weighed against the risks of barotrauma and reduced cardiac output. One series identified several subclinical cases of POPE8 that resolved without specific treatment. The role of diuretics in the management of POPE is unclear.