
A more recent article on vaginitis is available.
Am Fam Physician. 2000;62(5):1095-1104
Vaginitis is the most common gynecologic diagnosis in the primary care setting. In approximately 90 percent of affected women, this condition occurs secondary to bacterial vaginosis, vulvovaginal candidiasis or trichomoniasis. Vaginitis develops when the vaginal flora has been altered by introduction of a pathogen or by changes in the vaginal environment that allow pathogens to proliferate. The evaluation of vaginitis requires a directed history and physical examination, with focus on the site of involvement and the characteristics of the vaginal discharge. The laboratory evaluation includes microscopic examination of a saline wet-mount preparation and a potassium hydroxide preparation, a litmus test for the pH of vaginal secretions and a “whiff” test. Metronidazole is the primary treatment for bacterial vaginosis and trichomoniasis. Topical antifungal agents are the first-line treatments for candidal vaginitis.
Vaginitis is the most frequent gynecologic diagnosis encountered by physicians who provide primary care to women.1–5 Accurate diagnosis can be elusive, complicating treatment.6–9 Furthermore, the availability of over-the-counter medications increases the likelihood of inappropriate or partial treatment of vaginitis.10
Epidemiology
The prevalence and causes of vaginitis are uncertain, in part because the condition is so often self-diagnosed and self-treated. In addition, vaginitis is frequently asymptomatic or has more than one cause. Most experts believe that up to 90 percent of vaginitis cases are secondary to bacterial vaginosis, vulvovaginal candidiasis and trichomoniasis.4,8 Noninfectious causes include vaginal atrophy, allergies and chemical irritation.
Bacterial Vaginosis
In the United States, bacterial vaginosis is currently the most common cause of vaginitis, accounting for 40 to 50 percent of cases in women of childbearing age.11,12 This infection is believed to be caused by proliferation of a number of organisms, including Gardnerella vaginalis, Mobiluncus species, Mycoplasma hominis and Peptostreptococcus species.1,11
Determining the prevalence of bacterial vaginosis is difficult because one third to three quarters of affected women are asymptomatic.2,12,13 In addition, reported prevalences vary based on the population studied. Bacterial vaginosis has been found in 15 to 19 percent of ambulatory gynecology patients, 10 to 30 percent of pregnant patients and 24 to 40 percent of patients in sexually transmitted disease clinics.8,11–15
Even though higher rates of bacterial vaginosis have been reported in sexually transmitted disease clinics and in women with multiple sexual partners, the role of sexual transmission is unclear. Studies indicate that treating the male sexual partner of a woman with bacterial vaginosis is not beneficial and that even women who are not sexually active can have the infection.12,16 Additional risk factors for bacterial vaginosis include the use of intrauterine devices (IUDs), douching and pregnancy.1,11–13,17
Evidence suggests that bacterial vaginosis is a risk factor for premature rupture of membranes and preterm labor. Treating the infection in pregnancy decreases this risk.8,18–21 Additional possible adverse outcomes include an increased frequency of abnormal Papanicolaou (Pap) smears, pelvic inflammatory disease and endometritis.22–24 Vaginal cuff cellulitis, pelvic inflammatory disease and endometritis can occur if invasive gynecologic procedures or surgeries are performed when a patient has bacterial vaginosis.1,8,11
Vulvovaginal Candidiasis
Vulvovaginal candidiasis is the second most common cause of vaginitis in the United States and the most common cause in Europe.1 An estimated 75 percent of women have vulvovaginal candidiasis at some time in life, and approximately 5 percent of women have recurrent episodes.21,25–27 Candida albicans is the infecting agent in 80 to 90 percent of patients.8,10 Recently, the frequency of non-albicans species (e.g., Candida glabrata) has increased, possibly secondary to greater use of over-the-counter antifungal products.10
Risk factors for uncomplicated vulvovaginal candidiasis have been difficult to determine.28 Studies have shown that the risk of this infection is increased in women who use oral contraceptive pills, a diaphragm and spermicide, or an IUD.16,29,30 Other risk factors include young age at first intercourse, intercourse more than four times per month and receptive oral sex.25,27,28,31,32 The risk of vulvovaginal candidiasis is also increased in some women who have diabetes, are pregnant or are taking antibiotics.25,28,33
Establishing Candida species as the cause of vaginitis can be difficult because as many as 50 percent of asymptomatic women have candidal organisms as part of their endogenous vaginal flora.26 Candidal organisms are not transmitted sexually, and episodes of vulvovaginal candidiasis do not appear to be related to the number of sexual partners.25,27,28 Treating the male partner is unnecessary unless he is uncircumcised or has inflammation of the glans of the penis.36
Recurrent vulvovaginal candidiasis is defined as four or more episodes in a one-year period. It is not clear whether recurrences are secondary to predisposing and/or precipitating factors, sexual transmission, intestinal reservoir or vaginal persistence.37
Trichomoniasis
The protozoan Trichomonas vaginalis, a motile organism with four flagella,3 is the third most common cause of vaginitis. It affects 180 million women worldwide and currently accounts for 10 to 25 percent of vaginal infections.8 The incidence of trichomonal vaginitis is decreasing in most industrialized countries.1
Trichomonads are transmitted sexually and may be identified in 30 to 80 percent of the male sexual partners of infected women.8,38,39 Trichomoniasis is associated with and may act as a vector for other venereal diseases.40,41 Studies indicate that this infection increases the transmission rate of the human immunodeficiency virus.40
Risk factors for trichomoniasis include use of an IUD, cigarette smoking and multiple sexual partners.16,17,42 From 20 to 50 percent of women with trichomoniasis are asymptomatic.8,38 Trichomoniasis may be associated with premature rupture of membranes and preterm delivery.41 Sexual partners should be treated and instructed to avoid sexual intercourse until both partners are cured.36
Pathophysiology
The normal physiologic vaginal discharge comprises vaginal secretions, exfoliated cells and cervical mucus. The frequency of vaginal discharge varies with age, menstrual cycle, pregnancy and use of oral contraceptives.
The normal vaginal environment is characterized by a dynamic interrelationship between Lactobacillus acidophilus and other endogenous flora, estrogen, glycogen, vaginal pH and metabolic by-products of flora and pathogens. L. acidophilus produces hydrogen peroxide, which is toxic to pathogens and keeps the healthy vaginal pH between 3.8 and 4.2. Vaginitis occurs because the vaginal flora has been altered by the introduction of pathogens or changes in the vaginal environment that allow pathogens to proliferate.
Antibiotics, contraceptives, sexual intercourse, douching, stress and hormones can change the vaginal environment and allow pathogens to grow.3,4 In bacterial vaginosis, it is believed that some inciting event decreases the number of hydrogen peroxide–producing L. acidophilus organisms.11 The resultant change in pH allows proliferation of organisms that are normally suppressed, such as G. vaginalis, M. hominis and Mobiluncus species.11,12 These organisms produce metabolic byproducts, such as amines, that further increase the vaginal pH and cause exfoliation of vaginal epithelial cells. The amines are also responsible for the characteristic malodorous discharge in bacterial vaginosis.
Similarly, changes in the vaginal environment, such as an increase in glycogen production in pregnancy or altered estrogen and progesterone levels from the use of oral contraceptives, enhance the adherence of C. albicans to vaginal epithelial cells and facilitate the germination of yeast.26,28 These changes may transform asymptomatic colonization into symptomatic infection. In patients with trichomoniasis, changes in estrogen and progesterone levels, as well as elevations of vaginal pH and glycogen levels, may enhance the growth and virulence of T. vaginalis.42
Evaluation
A patient who complains of vaginal discharge, itching, frequent urination and/or irritation should be evaluated for vaginitis (Figure 1). The first step is to obtain a directed history. The patient should be asked about specific symptoms and their duration, any previous diagnosis and previous treatment and its effects. A general medical review, dermatologic review, social history and contraceptive history can also be helpful.
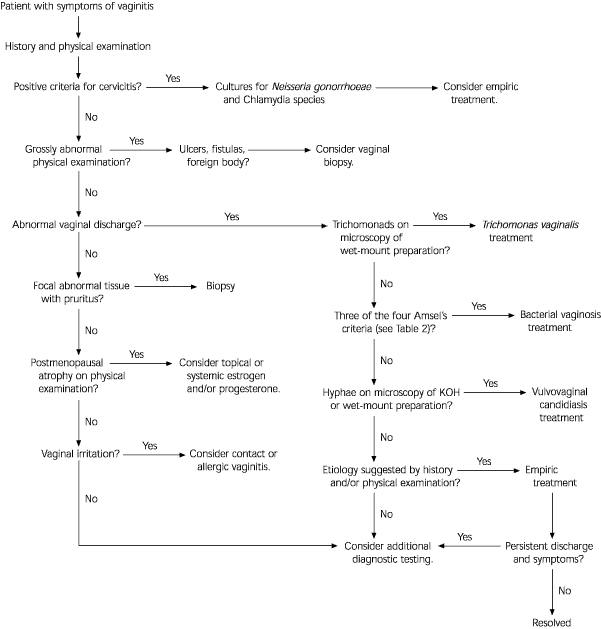
It is important to inquire about abdominal or pelvic pain, fever, recurrent or resistant infections, urinary symptoms, menstrual history, pregnancy and sexual practices.43 The nature of the discharge (i.e., amount, consistency, color, odor, accompanying pruritus) may also provide important clues. Dysuria is a common symptom of vaginitis. It is usually external and is defined as pain and burning when urine touches the vulva. In contrast, internal dysuria, defined as pain inside the urethra, is usually a sign of cystitis.5
A physical examination can help to identify the anatomic site of involvement (vulva, vagina or cervix). Inspection of the external genitalia for inflammation, lesions, masses, atrophic tissue and enlarged lymph nodes is important. The physician should also assess the patient for uterine or tubo-ovarian tenderness and perform a speculum examination to detect erythema, edema or lesions. The pooled vaginal discharge should be assessed for color, consistency, volume and adherence to the vaginal walls.
Because the diagnostic tests and treatments for cervicitis are different from those for vaginitis, it is important to differentiate these conditions. Several clues can help to rule out cervical infection as the cause of a vaginal discharge. Almost 90 percent of symptomatic or asymptomatic women with chlamydial cervicitis meet at least two of the following criteria: (1) younger than 24 years, (2) sexual intercourse with a new partner in the previous two months, (3) presence of mucopurulent cervicitis, (4) cervical bleeding induced by swabbing the endocervical mucosa and (5) no form of contraception.44 If cervicitis is suspected, cultures for Chlamydia species and Neisseria gonorrhoeae should be obtained.
If the findings of the history and/or physical examination suggest that the patient has vaginitis, a sample of the vaginal discharge should be obtained for gross and microscopic examination. Standard office examinations include a wet-mount preparation using saline, a slide prepared with 10 percent potassium hydroxide (KOH), a “whiff” test to detect amines and a litmus test of the pH level of vaginal fluid.
WET-MOUNT PREPARATION
A wet-mount preparation is obtained by diluting the vaginal discharge with one or two drops of 0.9 percent normal saline solution and placing it on a slide with a coverslip. Alternatively, the vaginal discharge can be put into a 2-mL test tube containing saline solution and then placed on a slide. The slide is examined microscopically using low power (10×) and high dry power (400×). The scanning of several fields for motile trichomonads has a sensitivity of 60 percent and a specificity of up to 99 percent.45
Microscopic examination of a wet-mount preparation can also detect “clue cells,” which are vaginal epithelial cells that are coated with the coccobacilli. When a skilled examiner performs the search for clue cells, examination of wet-mount preparations can have a sensitivity of 60 percent and a specificity of up to 98 percent for the detection of bacterial vaginosis.46–48 The examination may also detect fungal hyphae, increased numbers of polymorphonuclear cells (seen in trichomoniasis) or round parabasal cells (seen in atrophic vaginitis).
KOH PREPARATION AND WHIFF TEST
A second specimen of the vaginal discharge should be placed on a slide with a 10 percent KOH solution. A coverslip is placed on the slide and air- or flame-dried before examination under a microscope using low power.43 This is useful for detecting candidal hyphae, mycelial tangles and spores. The test is positive in 50 to 70 percent of women with candidal infection.49
During preparation of the KOH slide, a whiff test can be performed. The whiff test is positive if a “fishy” or amine odor is detected when KOH is added to the vaginal discharge. The odor results from the liberation of amines and organic acids produced from the alkalization of anaerobic bacteria. A positive whiff test is suggestive of bacterial vaginosis.11
LITMUS TESTING FOR PH
The pH level can be determined by placing litmus paper in the pooled vaginal secretions or against the lateral vaginal wall. The color is then compared to the colors and corresponding pH values on a standard chart. A normal vaginal pH is between 3.8 and 4.2.
Differential Diagnosis
The diagnosis of vaginitis is based on the patient's symptoms, the physical examination, the findings of microscopic examination of the wet-mount and KOH preparations, and the results of the pH litmus test. The features of bacterial vaginosis, vulvovaginal candidiasis and trichomoniasis are presented in Table 1.3,8 Women with bacterial vaginosis have a broad spectrum of clinical presentations. The classic presentation is a vaginal discharge with its characteristic odor and a clinical examination that is otherwise normal.
| Basis of diagnosis | Bacterial vaginosis | Vulvovaginal candidiasis | Trichomoniasis | |
|---|---|---|---|---|
| Signs and symptoms | Thin, off-white discharge Unpleasant “fishy” odor, with odor increasing after sexual intercourse | Thick, white (“cottage cheese”) discharge with no odor Pruritus Dysuria | Copious, malodorous, yellow- green (or discolored) discharge Pruritus Vaginal irritation No symptoms in 20 to 50 percent of affected women | |
| Physical examination | Usually, normal appearance of tissue; discolored discharge with abnormal odor, homogeneous discharge that adheres to vaginal walls | Vulvar and vaginal erythema, edema and fissures Thick, white discharge that adheres to vaginal walls | Vulvar and vaginal edema and erythema “Strawberry” cervix in up to 25 percent of affected women Frothy, purulent discharge | |
| Laboratory tests | ||||
| Vaginal pH (normal = < 4.5) | Elevated (> 4.5) | Normal | Elevated (> 4.5) | |
| Microscopic examination of wet-mount and KOH preparations of vaginal discharge | “Clue cells” (vaginal epithelial cells coated with coccobacilli) Few lactobacilli Occasional motile, curved rods (Mobiluncus species) | Pseudohyphae, mycelial tangles or budding yeast cells | Motile trichomonads Many polymorphonuclear cells | |
| “Whiff” test (normal = no odor) | Positive | Negative | Can be positive | |
| Additional tests | Amsel's criteria (three of four criteria must be met): provides correct diagnosis in 90 percent of affected women | KOH microscopy Gram stain Culture | DNA probe tests: sensitivity of 90 percent and specificity of 99.8 percent | |
| Culture: sensitivity of 98 percent and specificity of 100 percent | ||||
| Criteria of Nugent or Spiegel for Gram stain to diagnose bacterial vaginosis Other tests are controversial. | ||||
| Thin, homogeneous discharge |
| Positive “whiff” test |
| “Clue cells” present on microscopy† Vaginal pH > 4.5 |
Other investigators have developed criteria to diagnose bacterial vaginosis using a gram-stained vaginal smear3,50–52 (Tables 350 and 4)51. Evaluations of tests for bacterial vaginosis have shown that the Gram stain is better than gasliquid chromatography, G. vaginalis cultures or an assay for proline aminopeptidase.48
| Scoring system (zero to 7+)* is a weighted combination of the following bacterial morphotypes: | |
| A. Lactobacillus acidophilus (large gram-positive rods) B. Gardnerella vaginalis and Bacteroides species (small gram-variable or gram-negative rods) C. Mobiluncus species (curved gram-variable rods) | |
| The total score is the sum of the weighted quantity of the three bacterial morphotypes. | |
| Scoring for each of the above bacterial morphotypes: | |
| Zero = No morphotypes per oil-immersion field | |
| 1+ = Less than one morphotype per oil-immersion field 2+ = One to four morphotypes per oil-immersion field 3+ = Five to 30 morphotypes per oil-immersion field 4+ = More than 30 morphotypes per oil-immersion field | |
| Normal: Gram stain shows a predominance of Lactobacillus acidophilus (3+ or 4+), with or without Gardnerella vaginalis. | |
| Bacterial vaginosis: Gram stain shows mixed flora (gram-positive, gram-negative or gram-variable bacteria) and absent or decreased | |
| L. acidophilus (zero to 2+). A. L. acidophilus (large gram-positive bacilli) B. G. vaginalis (small gram-variable rods) | |
| Scoring for each of the above bacterial morphotypes: | |
| Zero = No morphotypes per oil-immersion field | |
| 1+ = Less than one morphotype per oil-immersion field 2+ = One to five morphotypes per oil-immersion field 3+ = Six to 30 morphotypes per oil-immersion field 4+ = More than 30 morphotypes per oil-immersion field | |
Women with vulvovaginal candidiasis frequently complain of pruritus, vaginal irritation and dysuria. Vulvovaginal itching generally is not a normal finding in healthy women; if this symptom is present, other dermatologic conditions (e.g., lichen sclerosis and, rarely, vulvar cancer) should also be considered, especially in the absence of candidal infection.5,43
In vulvovaginal candidiasis, the discharge is usually white and thick, with no odor and a normal pH. Women with candidiasis can have vulvar and vaginal erythema and, occasionally, scaling and fissures of vulvar tissue.8,49 Microscopic examinations of wet-mount and KOH preparations are positive in 50 to 70 percent of patients with candidal infections.49 In patients with a negative microscopic examination but symptoms compatible with yeast vaginitis, a Gram stain or a culture using Nickerson's medium or Sabouraud's dextrose agar may be helpful.4,5,28 Other tests, such as the Pap smear or the latex agglutination test, provide no advantage over microscopic techniques.21,26,53
For microscopic examination of the vaginal discharge, warming the slide and decreasing the intensity of substage lighting are ways to increase sensitivity for trichomonads.43 Additional testing may include cultures using Diamond's medium, which has a sensitivity of 95 percent for the diagnosis of trichomoniasis. Tests using DNA probes and polymerase chain reaction tests have high sensitivity and specificity.54 Cultures or other tests should be performed when the index of suspicion for trichomoniasis is high and examination of the wet-mount preparation is negative.
| Treatment regimens | Bacterial vaginosis | Vulvovaginal candidiasis | Trichomoniasis |
|---|---|---|---|
| Acute regimens | Metronidazole (Flagyl), 500 mg orally twice daily for seven days* | Topical antifungal agents† (see Table 6) Fluconazole (Diflucan), 150 mg orally one time | Metronidazole, 2 g orally in a single dose‡ |
| Clindamycin phosphate vaginal cream 2 percent (Cleocin Vaginal), one full applicator (5 g) intravaginally each night for 7 days* (Note that oil-based cream may weaken condoms and diaphragms.) | |||
| Metronidazole gel 0.75 percent (Metrogel-Vaginal), one full applicator (5 g) intravaginally twice daily for 5 days* | |||
| Alternative regimens | Metronidazole, 2 g orally in a single dose Clindamycin (Cleocin), 300 mg orally twice daily for 7 days | Boric acid powder in size-0 gelatin capsules intravaginally once or twice daily for 2 weeks§ | Metronidazole, 500 mg orally twice daily for 7 days |
| Pregnancy | Metronidazole, 250 mg orally three times daily for 7 days (recommended regimen)∥ | Only topical azole agents intravaginally for 7 to 10 days | Metronidazole, 2 g orally in a single dose (usually not recommended in first trimester) |
| Alternative regimens for pregnancy | Metronidazole, 2 g orally in a single dose∥ Clindamycin, 300 mg orally twice daily for 7 days∥ | ||
| Metronidazole gel 0.75 percent, one full applicator (5 g) intravaginally twice daily for 5 days (acceptable only in women who have not had a previous premature delivery) | |||
| Recurrence¶ | Retreat with an alternative regimen. | For four or more episodes of symptomatic vulvovaginal candidiasis annually: initial acute intravaginal regimen for 10 to 14 days followed immediately by maintenance regimen for at least 6 months (e.g., ketoconazole [Nizoral], 100 mg orally once daily) | Metronidazole, 2 g orally once daily for 3 to 5 days (Note that treatment of sexual partners increases cure rate.) |
| Butoconazole 2 percent cream (Femstat 3, Mycelex-3), 5 g per day intravaginally for 3 days*† |
| Clotrimazole 1 percent cream (Mycelex-7), 5 g per day intravaginally for 7 to 14 days*† |
| Clotrimazole 100-mg vaginal tablet (Gyne-Lotrimen, Mycelex), one tablet per day intravaginally for 7 days* |
| Clotrimazole 100-mg vaginal tablet, two tablets per day intravaginally for 3 days* |
| Clotrimazole 500-mg vaginal tablet (Mycelex-G), one tablet intravaginally in a single application* |
| Miconazole 2 percent cream (Monistat 7), 5 g per day intravaginally for 7 days*† |
| Miconazole 200-mg vaginal suppository (Monistat 3), one suppository per day for 3 days*† |
| Miconazole 100-mg vaginal suppository (Monistat 7), one suppository per day for 7 days*† |
| Nystatin 100,000-unit vaginal tablet (Mycostatin), one tablet per day intravaginally for 14 days |
| Tioconazole 6.5 percent ointment (Vagistat-1), 5 g intravaginally in a single application*† |
| Terconazole 0.4 percent cream (Terazol 7), 5 g per day intravaginally for 7 days* |
| Terconazole 0.8 percent cream (Terazol 3), 5 g per day intravaginally for 3 days* |
| Terconazole 80-mg vaginal suppository (Terazol 3), one suppository per day for 3 days* |