
Am Fam Physician. 2000;62(7):1565-1572
Anemia should not be accepted as an inevitable consequence of aging. A cause is found in approximately 80 percent of elderly patients. The most common causes of anemia in the elderly are chronic disease and iron deficiency. Vitamin B12 deficiency, folate deficiency, gastrointestinal bleeding and myelodysplastic syndrome are among other causes of anemia in the elderly. Serum ferritin is the most useful test to differentiate iron deficiency anemia from anemia of chronic disease. Not all cases of vitamin B12 deficiency can be identified by low serum levels. The serum methylmalonic acid level may be useful for diagnosis of vitamin B12 deficiency. Vitamin B12 deficiency is effectively treated with oral vitamin B12 supplementation. Folate deficiency is treated with 1 mg of folic acid daily.
Anemia is common in the elderly and its prevalence increases with age.1–4 Using World Health Organization criteria for anemia (hemoglobin of less than 12 g per dL [120 g per L] in women and less than 13 g per dL [130 g per L] in men), the prevalence of anemia in the elderly has been found to range from 8 to 44 percent, with the highest prevalence in men 85 years and older.1–3
The increased incidence of anemia with aging has led to speculation that lower hemoglobin levels may be a normal consequence of aging. However, there are at least two reasons for considering anemia in the elderly as a sign of disease. First, most older people maintain a normal red cell count, hemoglobin and hematocrit. Second, in most elderly patients an underlying cause of anemia is found for hemoglobin levels of less than 12 g per dL.5
Clinical Presentation
Even though the high prevalence of anemia in the elderly makes it a condition that clinicians might expect to find frequently, several features of anemia make it easy to overlook. The onset of symptoms and signs is usually insidious, and many elderly patients adjust their activities as their bodies make physiologic adaptations for the condition. Typical symptoms of anemia, such as fatigue, weakness and dyspnea, are not specific and in elderly patients tend to be attributed to advancing age. Pallor can be a helpful diagnostic clue, but pallor can be hard to detect in the elderly. Conjunctival pallor is a reliable sign, and its presence should prompt the clinician to order blood tests for anemia.6
Aside from conjunctival pallor, few other signs are attributable specifically to anemia. Frequently, patients have signs of a disorder that is made worse by the anemia, such as worsening congestive heart failure, cognitive impairment, dizziness and apathy. Unless clinicians consider anemia as a possibility in the elderly, it can be easily overlooked.
| Cause of anemia | Percentage of cases |
|---|---|
| Anemia of chronic disease | 30 to 45 |
| Iron deficiency | 15 to 30 |
| Posthemorrhagic | 5 to 10 |
| Vitamin B12 and folate deficiency | 5 to 10 |
| Chronic leukemia or lymphoma | 5 |
| Myelodysplastic syndrome | 5 |
| No identifiable cause | 15 to 25 |
EVALUATION
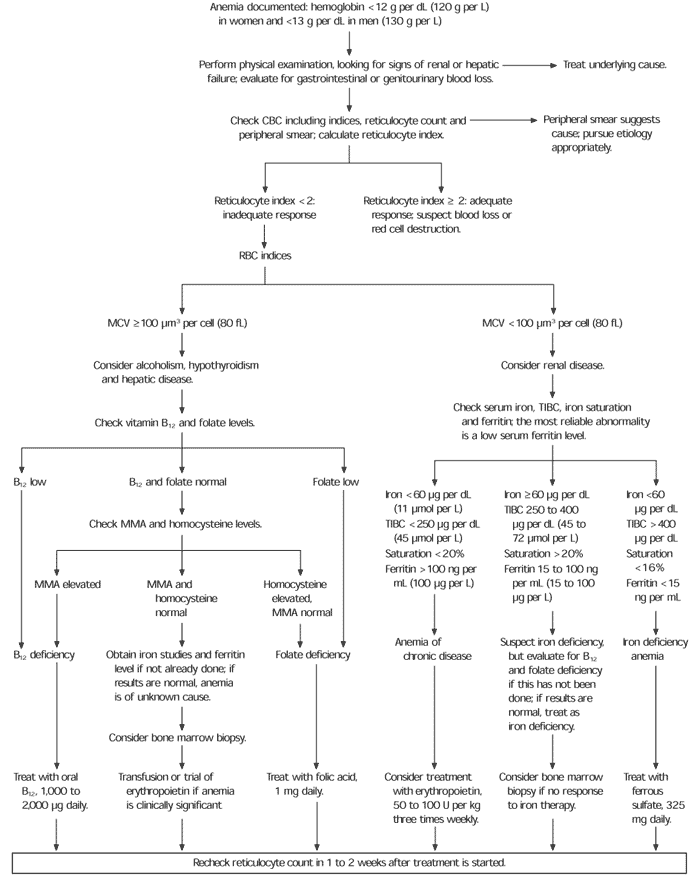
Anemia in the elderly is evaluated in a manner similar to that in younger adults, including an assessment for signs of gastrointestinal blood loss, hemolysis, nutritional deficiencies, malignancy, chronic infection (such as subacute endocarditis), renal or hepatic disease, and other chronic disease. In patients without evidence of an underlying disease, the initial laboratory evaluation should include a complete blood count, red blood cell indices, a reticulocyte count and peripheral blood smear (Table 2).
| Test | Finding | Possible etiology | |
|---|---|---|---|
| Complete blood count | |||
| RBC indices | MCV < 80 μm3 per cell (80 fL) | Iron deficiency anemia | |
| Anemia of chronic disease | |||
| MCV > 100 μm3 per cell (100 fL) | Vitamin B12 deficiency; folate deficiency | ||
| MCV normal | Renal, liver and thyroid diseases as well as those above | ||
| WBC and platelet counts | Abnormal | Primary marrow production problem | |
| Peripheral smear | Burr cells | Chronic renal failure | |
| Spherocytes, fragments | Hemolytic diseases | ||
| Dysplastic changes | Myelodysplasia | ||
| Reticulocyte count | < 1% | Inadequate production in presence of anemia | |
| ≥1% | Increased production but unclear whether it is of appropriate magnitude; reticulocyte index helpful for clarification | ||
| Reticulocyte index* | ≥2 | Reticulocyte release appropriate for anemia | |
| < 2 | Inadequate response to anemia | ||
Anemia algorithms used for evaluation of younger adults are based on the mean corpuscular volume. Such algorithms may be less helpful in the elderly because the classic changes in erythrocyte size do not often accompany anemia in this age group. In most elderly patients with anemia, red cell indices disclose normocytic, normochromic anemia.7,8 Clinicians therefore might begin the evaluation of anemia as they would in younger adults, but, if they do not find one of the classic causes of microcytosis or macrocytosis, the search for a cause might need to be enlarged (Figure 1). It also should be remembered that the cause of anemia is not always found.
Anemia of Chronic Disease
The hematologic abnormality in anemia of chronic disease is an impaired ability to use the iron stored in the reticuloendothelial system. The reason the reticuloendothelial cells do not release iron is not known, but experts speculate that, similar to fever, this response aids the body's defense mechanisms.11 Iron that is held in the reticuloendothelial system is not available for bacterial growth. Nor is the iron available for erythropoiesis, which is the similarity between anemia of chronic disease and iron deficiency anemia. The difference, however, is that the iron stores are normal or increased in anemia of chronic disease.
Patients with anemia of chronic disease have mild to moderate anemia that tends to correlate in severity with the underlying disease, although the anemia rarely progresses to a hemoglobin below 10 g per dL (100 g per L). In anemia of chronic disease, the erythrocytes are usually normochromic and normocytic, but about one third of patients with anemia of chronic disease have microcytosis.8,12
DIFFERENTIATING ANEMIA OF CHRONIC DISEASE FROM IRON DEFICIENCY ANEMIA
Iron deficiency anemia is the main hematologic disorder to consider in the differential diagnosis of anemia of chronic disease (Table 49,13).14–16 Iron deficiency anemia and anemia of chronic disease are accompanied by a low serum iron level. Microcytosis may or may not be present in either disorder. The total iron-binding capacity (TIBC) tends to be increased when iron stores are diminished and decreased when they are elevated. In classic iron deficiency anemia, the TIBC is higher than 400 μg per dL (72 μmol per L). In anemia of chronic disease, the TIBC is usually below normal, not only because the iron stores are elevated but also because, as an acute-phase reactant, transferrin is reduced in the presence of acute and chronic stress.
| Laboratory test | Range for normal values | Iron deficiency anemia | Anemia of chronic disease |
|---|---|---|---|
| Serum iron, μg per dL (μmol per L) | 60 to 100 (11 to 18) | < 60 (11) | < 60 (11) |
| Total iron-binding capacity, μg per dL (μmol per L) | 250 to 400 (45 to 72) | > 400 (72) | < 250 (44) |
| Transferrin saturation, % | 20 to 60 | < 16 | < 20 |
| Serum ferritin, ng per mL (μg per L) | 100 to 300 (100 to 300) | < 100 (100) | > 100 (100) |
The serum ferritin level is the most useful test, differentiating anemia of chronic disease from iron deficiency anemia in 70 percent of patients.17 Ferritin can also be an acute-phase reactant in liver injury and in some types of tumor, raising the serum ferritin to normal levels even in the presence of iron deficiency. For some cases in which both iron deficiency and anemia of chronic disease are possible, bone marrow aspiration is often the only means of identifying the true cause of the anemia (Table 49,13).
TREATMENT
There is no specific therapy for anemia of chronic disease except to manage or treat the underlying disorder. Iron therapy is of no benefit. Erythropoietin may be helpful in some patients with anemia of chronic disease. The dosage is 50 to 100 U per kg three times a week. The dosage can be increased to 150 U per kg per dose if the response to a lower dose is inadequate.
Iron Deficiency Anemia
Iron deficiency anemia, the second most common cause of anemia in the elderly, usually results from chronic gastrointestinal blood loss caused by nonsteroidal anti-inflammatory drug–induced gastritis, ulcer, colon cancer, diverticula or angiodysplasia. Chronic blood loss from genitourinary tract cancer, chronic hemoptysis and bleeding disorders may result in iron deficiency but are much less common causes. Older persons may become iron deficient because of inadequate intake or inadequate absorption of iron. Without blood loss, anemia takes several years to develop.
The serum ferritin level is the most effective way to diagnosis iron deficiency anemia. When the serum ferritin is less than 15 ng per mL (15 μg per L), iron deficiency is virtually certain (Table 514–17). Iron deficiency is unlikely if the serum ferritin level is greater than 100 ng mL (100 μg per L). Although ferritin levels between 15 and 100 ng per mL are moderately predictive of iron deficiency anemia, patients with levels in this range may have iron deficiency anemia, anemia of chronic disease, or both. If it is important to determine which is present or if the patient does not respond to iron therapy, a bone marrow biopsy might be necessary to measure iron stores directly.
| Serum ferritin, ng per mL (μg per L) | Sensitivity (%) | Specificity (%) | Likelihood ratio* |
|---|---|---|---|
| < 100 (100) | 94 | 71 | 3.2 |
| < 45 (45) | 85 | 92 | 11.1 |
| < 15 (15) | 59 | 99 | 54.5 |
Iron deficiency anemia in the elderly almost always leads to an evaluation of the gastrointestinal tract as a possible source of bleeding. In 20 to 40 percent of patients, the source is in the upper gastrointestinal tract from peptic ulcer disease, gastritis, esophagitis or gastric cancer.18–20 The blood loss is in the colon in 15 to 30 percent of cases, most often caused by colon cancer, angiodysplasia, polyps or colitis.18–20 A few patients (1 to 15 percent) have blood loss from disorders in the upper and lower gastrointestinal tract.18–20 The source is not found in the remaining 10 to 40 percent of elderly patients with gastrointestinal blood loss.18–20 Long-term follow-up of elderly patients in whom the gastrointestinal source is not identified indicates that most often the anemia resolves or remains stable with iron replacement.21
TREATMENT
In addition to treatment of the cause of bleeding, iron supplementation should be initiated for the treatment of iron deficiency anemia. The usual recommended dose of elemental iron is 50 to 100 mg three times a day; however, a smaller amount of elemental iron, such as a single 325-mg tablet of iron sulfate, may minimize side effects and improve compliance.22 This dose, equivalent to approximately 97.5 mg of elemental iron, is usually sufficient to replace iron stores, albeit at a slower rate.
Reticulocytosis usually starts within a week of initiation of oral iron supplementation. If the reticulocyte count increases but the anemia does not improve, continued blood loss or inadequate iron absorption must be considered. Intravenous iron replacement can be helpful in patients with iron deficiency that fails to respond to oral replacement.
Vitamin B12 Deficiency
While studies suggest that vitamin B12 (cobalamin) deficiency is the cause of anemia in 5 to 10 percent of elderly patients, the actual prevalence of vitamin B12 deficiency is likely to be much higher in the elderly.4,23 Vitamin B12 deficiency is difficult to detect in the elderly. First, the symptoms and signs of vitamin B12 deficiency are not reliably present in the elderly. Only about 60 percent of patients with vitamin B12 deficiency are anemic.20 In addition, neurologic symptoms of B12 deficiency can develop before the patient becomes anemic.24 Second, although anemia due to vitamin B12 deficiency is usually macrocytic and megaloblastic, it can be normocytic or even microcytic. Third, serum B12 levels do not reliably reflect tissue B12 deficiency. Up to 30 percent of patients with low-normal serum vitamin B12 levels have anemia and neurologic disease.23 This observation has prompted a search for more reliable ways of detecting vitamin B12 deficiency.
Studies have shown that serum methylmalonic acid and homocysteine levels are sensitive for detecting subclinical vitamin B12 deficiency, virtually excluding vitamin B12 deficiency when they are normal.25 These tests have become more widely available in recent years, but remain expensive, costing approximately $150 for each test. A less-expensive spot urinary methylmalonic acid assay has been purported to be an alternative to the serum assay.26 In addition to the advantage of reduced cost, the urinary assay can be normalized to creatinine levels, allowing correction for renal insufficiency and dehydration, both known to cause methylmalonic acid elevation. The disadvantage of the urinary assay is that it is not readily available.
A question that remains unanswered is which elderly patients with anemia should be further evaluated for vitamin B12 deficiency when the serum B12 level is normal. Some authorities recommend that serum B12 screening be initially performed in all elderly patients, with further testing in those with a B12 level of less than 350 pg per mL (260 pmol per L).4 Until the effectiveness of different screening strategies is evaluated, clinicians need to use their own judgment to decide how to identify vitamin B12 deficiency before it leads to anemia or neurologic disease.
CAUSES OF VITAMIN B12 DEFICIENCY
Vitamin B12 deficiency rarely is the result of inadequate intake, except in persons who are strict vegans. A common cause is reduced intestinal absorption of vitamin B12. Pernicious anemia is a classic example of a disorder that causes reduced intestinal absorption of vitamin B12. With pernicious anemia, the lack of intrinsic factor results from destruction of the gastric parietal cells by autoimmune antibodies. One study revealed that undiagnosed pernicious anemia was present in nearly 2 percent of otherwise healthy individuals 60 years or older.27 Inadequate absorption of vitamin B12 occurs in 10 to 30 percent of patients who have had a partial gastrectomy.28 It also may occur in patients with small bowel disorders and bacterial overgrowth. The prevalence of many of these conditions increases with age.
Previously, the Schilling test would have been used to identify the cause of inadequate vitamin B12 absorption. Recent studies, however, show that high-dose oral vitamin B12 effectively treats the deficiency regardless of the cause. Thus, there is less need to perform the Schilling test. (A review of the steps of the Schilling test can be found in a 1996 article in American Family Physician.29)
TREATMENT
Vitamin B12 deficiency is treated by vitamin B12 supplementation, parenterally or orally. The intramuscular dose is 1,000 μg, often given daily for one week to build up stores, then weekly for one month and then monthly thereafter. Oral therapy with 1,000 to 2,000 μg of vitamin B12 daily has been shown to be as effective as intramuscular injections and in some ways may be superior.26 A response to therapy, characterized by an increase in reticulocytosis, often occurs within a week of the initiation of vitamin B12 therapy.
Folate Deficiency
Unlike vitamin B12 deficiency, folate deficiency usually develops as a result of inadequate dietary intake. The body stores very little folate, only enough to last four to six months. Like vitamin B12 deficiency, folate deficiency classically causes macrocytic anemia, although a significant proportion (25 percent) of elderly patients with folate deficiency have normocytic anemia.8 The symptoms of folate deficiency are nearly indistinguishable from those of vitamin B12 deficiency.
Another similarity between folate deficiency and vitamin B12 deficiency is that the serum folate level can be misleading. The red cell folate concentration is more reliable than the serum level and should be considered. The serum homocysteine level is elevated in 90 percent of patients with folate deficiency25 and can be useful for detecting folate deficiency in patients with low-normal serum folate levels. If the methylmalonic acid level is also elevated, vitamin B12 deficiency must be considered.24 Identification of vitamin B12 deficiency is important: anemia secondary to vitamin B12 deficiency improves with folate therapy, but folate therapy does not reverse the neurologic damage caused by vitamin B12 deficiency. For this reason, it is important to ensure that vitamin B12 deficiency is not also present.
Folate deficiency is treated with oral folic acid, 1 mg daily.
Myelodysplastic Syndrome
Myelodysplastic syndrome is a relatively uncommon cause of anemia, but is a more common cause in the elderly than in younger patients. The syndrome, thought in the past to represent pre-leukemia, is characterized by a defect in the development of one of the marrow cell lines, limiting the release of functioning cells. Anemia results when the red cell lines are affected. Myelodysplastic syndrome should be a diagnostic consideration when white cell or platelet abnormalities accompany the anemia. The diagnosis of this syndrome is usually made by bone marrow biopsy (Table 64,22).
Myelodysplasia is treated supportively with transfusions.
| Pancytopenia |
| Monoclonal gammopathy |
| Suspicion of myelodysplastic syndrome |
| Blood smear showing immature white cells or nucleated red cells |
| Indeterminate status of iron stores |
| Unexplained progressive or unresponsive anemia |