
Am Fam Physician. 2000;62(7):1607-1612
See related patient information handout on taking methotrexate, written by the authors of this article.
Methotrexate has a long history of use in the treatment of various immunologic diseases, including rheumatoid arthritis and psoriasis. Although the drug is usually prescribed by a subspecialist, a family physician may assume responsibility for monitoring methotrexate therapy. Major toxic effects, such as hepatic, pulmonary, renal and bone marrow abnormalities, require careful monitoring. Minor toxic effects, such as stomatitis, malaise, nausea, diarrhea, headaches and mild alopecia, are common but respond to folate supplementation. Methotrexate is administered once weekly as a single dose or in divided doses given over a 24-hour period. To reduce the incidence of major toxic effects, methotrexate should never be given in daily doses. Relative contraindications include renal dysfunction, liver disease, active infectious disease and excessive alcohol consumption. Both women and men of reproductive age should use birth control during methotrexate therapy. Potential drug interactions include salicylates and nonsteroidal anti-inflammatory drugs, which are both commonly used in patients with rheumatoid arthritis or psoriasis. A premethotrexate evaluation is important to ensure proper patient selection for this effective but potentially toxic drug.
Methotrexate has an unusual history. What began as a drug for the treatment of cancer, particularly childhood leukemia, is now used to treat a wide variety of immunologic disorders.1 The use of methotrexate in the treatment of psoriasis and rheumatoid arthritis dates from the 1960s.1,2 From the late 1970s to the early 1980s, many rheumatologists were reporting their experiences with methotrexate use in studies of rheumatoid arthritis.2 Guidelines for methotrexate use were then developed to address dosing, liver biopsy and monitoring strategies, which were aimed at reducing the incidence of adverse effects.2 Currently, methotrexate is indicated for the treatment of acute lymphocytic leukemia (ALL), rheumatoid arthritis and psoriasis. However, the drug has also been found efficacious in the treatment of other diseases, including asthma, systemic lupus erythematosus, Crohn's disease, myositis, vasculitis and ectopic pregnancy.3–5 Many physicians use methotrexate for its steroid-sparing properties in patients with asthma and others who may have side effects related to corticosteroid use.6 The key to the success of methotrexate in treating any of these diseases (with the exception of ALL) is the recognition that low-dose therapy achieves efficacy while minimizing side effects.
It is not uncommon for a family physician to monitor the efficacy and safety of methotrexate therapy in a patient receiving concurrent care from the subspecialist who prescribed the drug.
Minor and Major Toxic Effects
Methotrexate is a toxic medication, but if it is dosed correctly and monitored appropriately, its toxic effects can be minimized.7 These effects are categorized as minor or major.
Minor toxic effects such as stomatitis, malaise, nausea, vomiting, diarrhea, headaches and mild alopecia are not life threatening but occur in 20 to 30 percent of patients. Other effects in this category include fatigue, mood alteration, dizziness, fever, myalgias and polyarthralgia. Most minor toxic effects are associated with depletion of folate. Folate supplementation with 1 mg daily or 7 mg once weekly should be considered for all patients.8 Studies show that low-dose folate does not interfere with the efficacy of methotrexate.9 Most rheumatologists advise patients to avoid taking the folate dose on the same day as the methotrexate dose. Often, minor toxic effects respond to a reduction in the methotrexate dose or an adjustment in the dosing schedule.
Rheumatoid nodules may also increase in size during methotrexate therapy, despite good control of the disease process.10
Major toxic effects of methotrexate, such as hepatic, renal, pulmonary and bone marrow disorders, occur less frequently than the minor effects but may be life threatening.7 (A methotrexate monitoring form is shown in Figure 1. It may be copied and used as part of the patient record for data collection and assessment.) Patients should be warned of the possible development of malignant hematologic diseases such as non-Hodgkin's lymphoma during therapy. The methotrexate package insert cites cases in which malignant lymphoma regressed after withdrawal of methotrexate without the need for antilymphoma treatment. Appropriate risk assessment is required to ensure selection of the proper candidate for methotrexate therapy.
HEPATOTOXICITY
Although not appreciably metabolized, methotrexate concentrates in the liver. Abnormal liver histology appears to be more common in patients with psoriasis than in those with rheumatoid arthritis.7 Patients may experience a range of liver problems from mild fatty infiltrate to moderate or severe fibrosis, necrosis and cirrhosis.8 Hepatotoxic effects are associated with long-term use and high doses of methotrexate and are common in patients taking a daily dose, which is never advisable.7
Monitoring of serum aspartate aminotransferase (AST) and serum albumin levels is recommended for all patients receiving methotrexate.8 These laboratory assessments were correlated with serious liver disorders in a mail survey of rheumatologists.11 Approximately 30 percent of all patients on long-term therapy have AST elevation. Values exceeding two times the normal level for a period of one month warrant discontinuation of therapy.7
Because liver function tests do not always predict hepatotoxic effects from methotrexate use, a liver biopsy may be indicated under the following circumstances in patients with rheumatoid arthritis: persistent elevation in liver enzymes; abnormal results in five of nine determinations of AST levels within a 12-month period; and a decrease in serum albumin values below the normal range.8,12 Current studies in patients with rheumatoid arthritis suggest that liver biopsies are not cost-effective for at least the first 10 years of methotrexate use in patients with normal values on liver function tests.8
Routine surveillance liver biopsies are not recommended for rheumatoid arthritis patients who take the recommended dose.8
The dermatology literature, however, recommends a liver biopsy after a cumulative dose of 1.5 g in patients with psoriasis, including those who lack significant risk factors for hepatic disease.13 These guidelines have been challenged by Australian dermatologists citing a 2.2 percent complication rate from liver biopsy and a mortality rate of nine per 100,000 biopsies.14 There seems to be much more disagreement among dermatologists than rheumatologists concerning the need for liver biopsy.
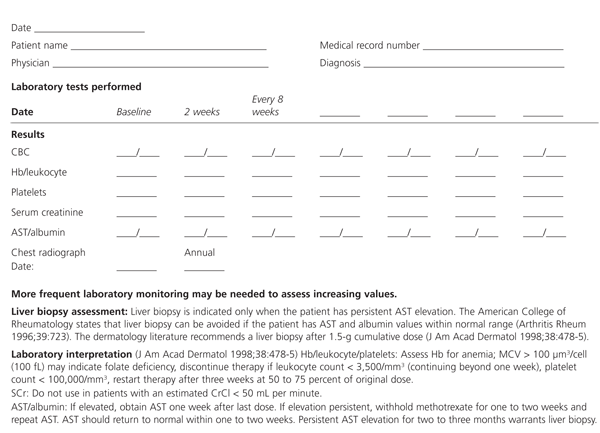
A premethotrexate liver biopsy is indicated for patients who have a history of excessive alcohol intake, elevated AST values or chronic hepatitis B or C.7,12 Other factors that may increase the risk of hepatotoxic effects are included in Table 1.7,8,13 The dermatology literature recommends performing a liver biopsy in the first four months of therapy in patients with significant risk factors.13
| Excessive alcohol intake |
| Elevated serum aspartate aminotransferase levels |
| Chronic hepatitis B or C |
| Increased age |
| History of liver disease |
| History of intravenous drug abuse |
| History of inheritable liver disease |
| Diabetes (insulin enhances cytotoxicity of methotrexate) |
| Obesity (third spacing of methotrexate) |
| History of significant exposure to known hepatotoxic drugs |
NEPHROTOXICITY
Methotrexate concentrates in the kidneys, gallbladder and spleen as well as in the liver. Renal excretion eliminates 60 to 95 percent of a dose. Tubular secretion, reabsorption and glomerular filtration are all involved in the renal elimination of methotrexate. Therefore, methotrexate is contraindicated in any patient with a creatinine clearance of less than 50 mL per minute.13
PULMONARY TOXICITY
Pulmonary abnormalities are emerging as the more common major toxic effects of methotrexate use (5 percent incidence).7 Symptoms include persistent dry, nonproductive cough, dyspnea or both. Patients may also have fever. Pulmonary toxicity is unrelated to the cumulative dose and has occurred in early and late stages of therapy.7 In one case-control study, increased age was the strongest predictor of lung injury.15 The clinical trials have revealed a higher incidence in patients with rheumatoid arthritis than in those with psoriasis.13 Pulmonary toxicity is also associated with a divided weekly dose regimen rather than a full weekly dose regimen.13
A baseline chest radiograph is recommended to screen for pre-existing lung disease.8 Radiograph abnormalities occurring with methotrexate therapy include interstitial and alveolar infiltrates, hilar adenopathy and pleural effusion, occasionally progressing to fibrosis, scarring and honeycomb changes.16,17 These changes can also occur in the rheumatoid lung and are sometimes indistinguishable from those resulting from methotrexate therapy.
BONE MARROW TOXICITY
Bone marrow effects are rare but are associated with the following conditions: high doses of methotrexate, underlying renal disease, infection, folate deficiency, increased age, low albumin and concurrent use of trimethoprim (an antifolate reductase inhibitor). Monitoring signs of myelosuppression can reduce complications such as severe anemia, potential bleeding and sepsis.8 Patients with aplastic anemia need to be treated with leucovorin rescue.13
Relative Contraindications
The decision to use methotrexate should be made by the patient and the physician, who should weigh the risks and benefits of therapy. Pregnancy and lactation are considered the only absolute contraindications. Both women and men of reproductive age should use birth control during methotrexate therapy and after discontinuation of therapy for one month in women and three months in men. Some physicians believe that alcohol consumption in any amount should be an absolute contraindication because no data suggest a safe consumption level.14 Recently, the FDA warned against using methotrexate concomitantly with radio-therapy. The combination increases the risk of soft tissue necrosis and osteonecrosis. The relative contraindications are listed in Table 2.13,15
| Renal dysfunction (dosage adjustments needed) |
| Significantly abnormal results on liver function tests |
| Hepatitis |
| Cirrhosis |
| Significant pulmonary disease |
| Blood dyscrasias (severe anemia, leukopenia, thrombocytopenia) |
| Excessive alcohol consumption |
| Active infectious disease (tuberculosis, pyelonephritis) |
| HIV or AIDS |
| Patient unreliability |
| Radiotherapy |
Drug Interactions
Preventing a reduction in renal elimination of methotrexate is the key to minimizing consequences from drug interactions. Salicylates and nonsteroidal anti-inflammatory drugs (NSAIDs) can decrease the renal elimination and the tubular secretion of methotrexate. These drugs can also displace methotrexate from protein binding sites, increasing serum levels of methotrexate. Because most patients with rheumatoid arthritis or psoriasis require an NSAID, it is important to titrate methotrexate carefully and monitor for efficacy and side effects.
Trimethoprim/sulfamethoxazole (Bactrim, Septra) can enhance the cytotoxic effects of methotrexate because trimethoprim is an antifolate reductase inhibitor. In addition to salicylates and NSAIDs, other drugs that may displace methotrexate from protein binding sites include barbiturates, phenytoin, retinoids, oral sulfonylureas and tetracycline.
Dosing and Availability
Methotrexate can be given orally or by intramuscular or subcutaneous injection.18 Intramuscular or subcutaneous administration is usually reserved for patients with poor oral bioavailability or poor adherence to oral therapy, or when cost is an issue.
Weekly dosing of methotrexate is recommended. The entire dose can be administered at once or divided into three doses taken over a 24-hour period (i.e., every eight hours). Methotrexate should never be given in daily doses. More frequent administration than weekly increases the risk of toxicity. Most patients show a therapeutic response with weekly doses of oral or injection therapy between 7.5 mg and 15.0 mg, although some patients may need 20 or even 30 mg, the maximum recommended dose. Therapeutic response usually begins at three to six weeks, and the patient may continue to improve over a 12-week period. Treatment should not be considered a failure unless the methotrexate doses have been escalated above 15.0 mg to 17.5 mg per week without a clinical response.2 Again, folate supplementation with 1 mg per day or 7 mg once weekly should be considered for all patients.8
Methotrexate is available in 2.5-mg tablets as a generic or a brand-name dose pack (Rheumatrex). The dose packs consist of four cards with two, three, four, five or six 2.5-mg tablets. The cost for a weekly dose (7.5 to 15.0 mg) of methotrexate tablets ranges from $6 to $15 for the generic product and from $9 to $22 for Rheumatrex. The weekly cost for methotrexate injection (generic only) ranges from $6 to $25.19
Evaluation for Methotrexate Therapy
A premethotrexate evaluation is important to ensure that the patient is a suitable candidate for therapy with the drug. The evaluation consists of a thorough history, which should address alcohol consumption as well as hepatic and renal risk. It is important to assess the expected reliability of the patient for physician visits and follow-up laboratory visits. If the patient is deemed unreliable, then injection of methotrexate at the physician's office should be considered to increase adherence to therapy and reduce toxicity. The American Academy of Dermatology consensus conference on the use of methotrexate in psoriasis recommends the tests listed in Table 3.13
| Complete blood count with differential |
| Platelet count |
| Serum creatinine |
| Blood urea nitrogen |
| Urinalysis |
| Liver function tests |
| Serum bilirubin |
| Serum albumin |
| Hepatitis A, B, and C serologies |
| HIV risk assessment/testing, if appropriate Chest radiograph |
Counseling the Patient Taking Methotrexate Therapy
It is important for the family physician to effectively counsel the patient who is taking methotrexate. Table 4 lists some patient education guidelines for effective counseling. In addition, the family physician and the prescribing physician (usually a rheumatologist or a dermatologist) must maintain communication in regard to laboratory testing and clinical monitoring for response and drug toxicity.
| Tell patients to avoid alcohol including beer, wine and hard liquor because of the increased risk of liver disease. |
| Inform male and female patients of reproductive age that they should practice appropriate birth control (abstinence, oral contraceptives or condom plus foam, etc.). |
| Discuss potential drug interactions, especially salicylates and over-the-counter NSAIDs. |
| Tell patients not to start or stop an NSAID without first checking with you. |
| Tell patients to call immediately if they develop signs of infection (immunosuppression), coughing or shortness of breath (pulmonary toxicity) or unusual bleeding (liver or bone marrow suppression). |
| Emphasize the weekly dose and warn patients that daily dosing of this drug is fatal. If an accidental overdose occurs, an antidote can be used (leucovorin rescue). |
| Be sure that patients fully understand the need for close follow-up and monitoring for toxicity. |
| The most important side effects to mention are loss of appetite, nausea (rarely vomiting), diarrhea and stomatitis. There is also the potential for serious side effects; hepatotoxicity, pulmonary toxicity, myelosuppression and nephrotoxicity. |
| Warn patients about the potential development of malignancy, specifically lymphoma. |