
Am Fam Physician. 2000;62(10):2287-2292
See related editorials: Informed Consent and Emergency Contraception and Emergency Contraception: Still Not Too Late.
Emergency postcoital contraception, a method used to prevent pregnancy after unprotected sexual intercourse, is a highly effective but underutilized birth control option. Two hormone regimens, ethinyl estradiol (100 μg) with levonorgestrel (0.5 mg) or high-dose levonorgestrel (0.75 mg), given within 72 hours of intercourse and repeated 12 hours later, are available for this purpose. These regimens are packaged as Food and Drug Administration labeled, dedicated products or can be adapted for use from standard oral contraceptive pills. Emergency postcoital contraception should be considered as a primary prevention health service to women of childbearing age.
The use of safe, effective emergency postcoital contraceptive methods could result in 1 million fewer abortions and 2 million fewer unintended pregnancies each year in the United States. Although 2 to 3 percent of women terminate a pregnancy each year through abortion, only 1 percent of women in America report ever having used emergency contraception.2 Studies of college women who have had abortions reveal that, on average, only one in three was aware that emergency contraception was available.
Worldwide, emergency contraception has been used extensively for over two decades. The options currently available include an estrogen-progestin combination (ethinyl estradiol with levonorgestrel), progestin alone (levonorgestrel), the antiprogestin synthetic steroid RU 486 (mifepristone; Mifeprex), estrogen alone (high-dose ethinyl estradiol) and the copper intrauterine device (IUD) (Table 1).3
Mechanism of Action
The precise mechanisms of these contraceptive agents are not completely known. Human and animal studies have shown effects at several stages of the reproductive cycle: ovulation, fertilization, gamete transport, function of the corpus luteum and implantation.3 Specific human data about exogenous hormone effect on each of these stages have been limited in the United States because the study processes required would violate the ethical guidelines for human subjects. Estrogen and progesterone, alone or in combination, inhibit or delay ovulation. However, in human studies the evidence is less clear about cumulative effects on fertilization, gamete transport, the endometrium, functioning of the corpus luteum or implantation. Histologic alterations and biochemical changes are demonstrated, although they may not be significant enough to prevent pregnancy at any given point in the cycle. Some individuals may consider these hormones to be abortifacients if they interfere with implantation. However, the proven mechanisms of action consist of inhibiting or delaying ovulation. These hormones will not dislodge an implanted embryo.
Mifepristone inhibits ovulation and blocks implantation by causing a delay in maturation of the endometrium.3 It causes actual regression of the corpus luteum in 50 percent of women when given in the middle or late luteal phase.
The copper IUD inhibits fertilization through its toxic effects on the sperm and blocks implantation through the effects of the foreign body and trace mineral release on a changing endometrium.
Only mifepristone is effective once implantation has occurred, actually interrupting an early pregnancy. The effectiveness of these methods thus depends on the point in a woman's reproductive cycle at which emergency contraception is used.
Physicians must understand the probability of conception when emergency contraception is considered. The average fertile period for a woman lasts only six days per menstrual cycle and ends the day she ovulates. Unprotected sex three days before ovulation results in an estimated 15 percent pregnancy rate; one or two days before ovulation, a 30 percent rate; and on the day of ovulation, an estimated 12 percent rate. Sperm can survive in the female up to five days, and the mature egg may be fertilized over a 24-hour period. The time period from ovulation to implantation is about seven days.4
Emergency Contraceptive Options
COMBINED ETHINYL ESTRADIOL AND LEVONORGESTREL
In 1977, Yuzpe and Lancee5 introduced a regimen for emergency contraception consisting of ethinyl estradiol, 100 μg, and levonorgestrel, 0.5 mg, to be taken within 72 hours of unprotected intercourse and repeated 12 hours later. This regimen remains the most commonly prescribed postcoital birth control method in the United States. A number of pills from different trade name birth control pill packages are used (Table 2). More recently, a prepackaged, dedicated product consisting of four pills (Preven) has been developed.
| Trade name | Pills per dose | Ethinyl estradiol per dose (μg) | Levonorgestrel per dose (mg) | Cost* |
|---|---|---|---|---|
| Ovral†‡ | 2 white pills | 100 | 0.50 | $47 |
| Alesse | 5 pink pills | 100 | 0.50 | 29 |
| Levlite | 5 pink pills | 100 | 0.50 | 28 |
| Nordette† | 4 light-orange pills | 120 | 0.60 | 30 |
| Levlen† | 4 light-orange pills | 120 | 0.60 | 30 |
| Levora | 4 white pills | 120 | 0.60 | 27 |
| Lo/Ovral†‡ | 4 white pills | 120 | 0.60 | 31 |
| Triphasil† | 4 yellow pills | 120 | 0.50 | 29 |
| Tri-Levlen† | 4 yellow pills | 120 | 0.50 | 28 |
| Trivora | 4 pink pills | 120 | 0.50 | 28 |
| Ovrette‡ | 20 yellow pills | 0 | 0.75 | 31 |
| Dedicated products | ||||
| Preven§∥ | 2 blue pills | 100 | 0.50 | 20 |
| Plan B∥ | 1 white pill | 0 | 0.75 | 20 |
If 100 women have unprotected intercourse during the second or third week of their cycle, the probability is that eight will become pregnant. If the Yuzpe method is used, only two women will become pregnant (about a 75 percent reduction). A recent review of eight studies showed a precise reduced risk of pregnancy of 74.1 percent (95 percent confidence interval, range: 62.9 to 79.2 percent).6
Common side effects include nausea (50 percent of women) and vomiting (20 percent). No studies have analyzed the effect of vomiting on the efficacy of this regimen. Some physicians prescribe antiemetics routinely with the hormone therapy or repeat the dose of medication if vomiting occurs within one to two hours of ingestion. Others theorize that if nausea is experienced, the medication must already be absorbed systemically. Less common side effects include heavy menses and mastalgia.
Withdrawal bleeding occurs within three weeks of treatment. Thirty-eight percent of women bleed before their menstrual period is due. Only 8 percent are estimated to be four or more days late.3
No data support the occurrence of vascular complications in women resulting from this brief use of estrogen and progesterone therapy.7 In England, where emergency contraception has been used in over 4 million cases in 13 years, no statistically significant increase in the rate of deep venous thrombosis has occurred.3
There are no absolute contraindications to emergency contraception other than pregnancy. Furthermore, studies have shown no evidence of harm to the developing fetus. Investigators have not specifically examined the teratogenic effects associated with emergency contraceptive use, although a reasonable extrapolation may be made from extensive experience with prospective studies of unintended pregnancy in oral contraceptive users.
It is not known whether using estrogen-progestin combinations other than those described by Yuzpe would also be effective, whether the second dose given 12 hours after the first dose is necessary or whether the pills would be effective if started later than 72 hours after intercourse. A recent analysis of the timing of pill use suggests an inverse linear relationship between efficacy and the time from intercourse to treatment. The earlier the pills were used, the more effective they were during the 72-hour period studied. Delaying the first dose by 12 hours increased the odds of pregnancy by almost 50 percent.8 This analysis did not, however, study effectiveness beyond 72 hours. (If a patient first contacts her physician more than 72 hours after unprotected intercourse takes place, the medication can still be prescribed, with reassurance that no harm will be done if she becomes pregnant and a discussion of the presumably reduced efficacy of this regimen beyond 72 hours.)
LEVONORGESTREL
Within the past few years, evidence has emerged to support the preferential use of the progestin-only regimen (levonorgestrel, 0.75 mg), given within 72 hours of intercourse and repeated 12 hours later.
The World Health Organization conducted a large, double-blind, randomized trial9 involving 1,998 women from 14 countries, comparing the use of levonorgestrel with the Yuzpe estrogen-progestin combination. The levonorgestrel regimen decreased the average expected pregnancy rate by 85 percent (95 percent confidence interval; range: 74 to 93 percent) compared with the Yuzpe regimen rate of 57 percent (95 percent confidence interval; range: 39 to 71 percent). Although the decreased rate of pregnancy with use of the Yuzpe regimen in this study was less than that in the earlier review of 11 studies,10 it was still within the range of pregnancy rates in previous individual studies.
This large trial also demonstrated an improved side effect profile of the levonorgestrel protocol. Only 23.1 percent of women using progestin alone experienced nausea compared with 50 percent of women using the combined regimen. Similarly, only 5.6 percent of women reported vomiting compared with 18.8 percent using the Yuzpe regimen. In both groups, the efficacy was inversely related to length of time from intercourse and commencement of medication within the 72-hour window studied.9,10 Menstrual patterns were similar in both study groups.
As in the Yuzpe regimen, the only absolute contraindication to the levonorgestrel protocol is pregnancy, because the medication would be ineffective.
Levonorgestrel in a two-pill sequential dosing packet (Plan B) is available in the United States and is labeled by the FDA for emergency contraceptive use. The more cumbersome regimen of 20 levonorgestrel pills (Ovrette) per dose may also be prescribed.
MIFEPRISTONE
Two randomized trials have compared 600 mg of mifepristone with the Yupze regimen.11,12 In these trials, one 600-mg dose given within 72 hours of unprotected intercourse was 100 percent effective as an emergency contraceptive. Nausea and vomiting were less common than with the Yuzpe method. (No studies have specifically compared mifepristone with levonorgestrel.) However, delay of menses was more common with mifepristone; 42 percent of women experienced a delay of more than three days.
A recent multicenter, randomized trial involving 1,700 women studied the effectiveness of lower doses of mifepristone.13 Either 600-mg, 50-mg or 10-mg doses were given one time only within five days of unprotected intercourse. These three regimens all decreased the pregnancy rate by about 85 percent. However, the delay in the onset of menses was significantly related to the dosage, with menstrual delay of more than one week in 36 percent of women taking 600 mg, in 23 percent of women ingesting 50 mg, and in only 18 percent after a 10-mg dose. The 10-mg dose appears to be equally effective with fewer side effects.
Mifepristone is currently available for use by physicians in the United States, having been approved as an abortifacent by the FDA in October. It can legally be prescribed off-label as a “morning-after” pill. However, it is available in this country only in the 200-mg concentration, a significantly higher dose than that required for emergency postcoital contraception according to international trials.
INTRAUTERINE DEVICE
The copper IUD, which has been used extensively in European countries, is a second-line, highly effective form of postcoital contraceptive. Estimated failure rates are less than 1 percent. Because this method prevents implantation, an IUD may be inserted up to five days after the earliest estimated day of ovulation. If a woman is planning to use an IUD for future contraception, introducing it as an emergency contraceptive is a reasonable solution. The IUD's side effect of increased pelvic infections in women with multiple partners or those with an active history of sexually transmitted disease and its current rare use in nulliparous women make its applicability more limited.
Clinical Decision-Making
The indications for emergency contraception are listed in Table 3.
| Intercourse without contraceptive protection, including unsuccessful withdrawal before ejaculation or ejaculation on external genitalia |
| Barrier method inserted incorrectly, dislodged, torn during intercourse or removed too early |
| Inadvertent expulsion or partial expulsion of intrauterine device |
| Failure to take multiple birth control pills (three or more) in two weeks preceding intercourse |
| Exposure to possible teratogen, such as live vaccine, cytotoxic drug, extensive radiographic studies |
| Sexual assault |
The date of the patient's last menses, her contraceptive history and the dates of unprotected intercourse should be determined. The patient's likely risk of pregnancy should be discussed, as well as her feelings about continuing the pregnancy in the event that emergency treatment is not effective.
The physician should decide whether a limited physical examination or laboratory testing is indicated (Table 4). If there is doubt about exposure to unprotected intercourse during the previous cycle, a pregnancy test should be performed. Evaluation for sexually transmitted disease should be considered in patients who are at risk.
| Suspected pregnancy |
| Assessment for sexually transmitted disease |
| Consideration of insertion of intrauterine device |
| Overdue Papanicolaou smear |
The patient should be advised that 98 percent of women will bleed within 21 days of use of emergency contraception. If bleeding does not occur within four weeks of emergency contraception, a pregnancy test should be performed. If the physician wishes to schedule a follow-up visit, it should be scheduled for three weeks after the emergency contraceptive is used.
Most importantly, patients should be counseled to consistently use a precoital method of birth control. The failure rates of emergency postcoital contraception accumulate over time, and this method is not as effective as precoital birth control. (When used correctly, oral contraceptives have a 99 percent rate of efficacy.) Physicians should take this opportunity to educate women about more effective options.
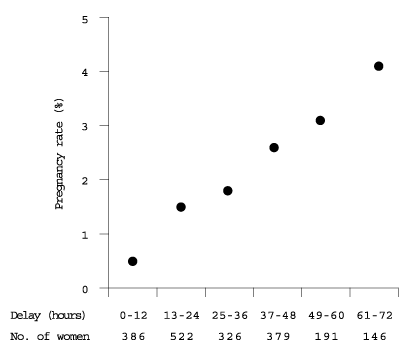
Emergency contraception should be discussed at all preventive health visits focusing on sexuality and birth control. The physician should consider whether a prescription for emergency postcoital treatment should be offered to the patient at preventive health visits. The data indicate that the earlier the hormone medication is taken, the less likely a subsequent pregnancy (Figure 1).8 Rapid access is an issue, especially since some women may need emergency contraception more than once during the lifetime reproductive cycle. Physicians who counsel patients wisely and are clear about follow-up can feel comfortable that this option is not likely to be abused. One recent randomized study14 involving 1,000 women in Scotland demonstrated that those who self-administered emergency contraceptive pills were no more likely than other women to use emergency contraception repeatedly or to make less use of other contraceptive methods.
The two new dedicated products, Preven and Plan B, are not yet widely available. Plan B is, as of mid-October, newly available for purchase in pharmacies via physician prescription, having previously been distributed only in Title 10 clinics, Planned Parenthood clinics, state and county health departments and university campus health centers. Preven has been available through pharmacies for over a year, but still is not readily available in many locations. Both dedicated products should become more accessible in all locations according to physician demand.
Physicians may also prescribe the standard, appropriate oral contraceptives or work with pharmacists to break up pill packages to provide the exact number of pills. In some programs across the country, pharmacists are licensed to provide a limited number of emergency contraceptive pills on an urgent basis without previous physician approval.15 Physicians must determine the most effective strategy for their local community.
Family physicians should inform their patients of reproductive age about the availability and efficacy of emergency postcoital contraception. Patient-centered education and decision-making about therapeutic options can only enhance the care of women faced with the possible crisis of an unplanned pregnancy.