
Am Fam Physician. 2001;63(3):483-491
See patient information handout on plasma viral load testing and HIV, written by the authors of this article.
The polymerase chain reaction assay, branched DNA assay and nucleic acid sequence-based amplification assay quantitate human immunodeficiency virus (HIV) RNA levels. Plasma viral load (PVL) testing has become a cornerstone of HIV disease management. Initiation of antiretroviral drug therapy is usually recommended when the PVL is 10,000 to 30,000 copies per mL or when CD4+ T-lymphocyte counts are less than 350 to 500 per mm3 (0.35 to 0.50 × 109 per L). PVL levels usually show a 1- to 2-log reduction within four to six weeks after therapy is started. The goal is no detectable virus in 16 to 24 weeks. Periodic monitoring of PVL is important to promptly identify treatment failure. When feasible, the same assay should be used for serial PVL testing in the individual patient. At least two PVL measurements usually should be performed before antiretroviral drug therapy is initiated or changed. PVL testing may be helpful in the rare instance of indeterminate HIV antibody testing, especially in a patient with recent infection.
In the 1990s, new technologies, including the polymerase chain reaction (PCR) assay,1 the branched DNA (bDNA) assay2 and the nucleic acid sequence-based amplification (NASBA) assay,3 made it possible to obtain accurate quantitative measurements of human immunodeficiency virus (HIV) RNA in plasma. HIV RNA plasma levels have proved to be a powerful predictor of risk for disease progression.1,4,5 They are also commonly employed to determine the relative effectiveness of antiretroviral drugs in clinical trials.1,4,5
The use of HIV RNA assays alerted investigators to the extraordinary rate of viral replication in vivo, debunking earlier theories that the initial years of HIV infection were a relatively “benign” period. The increasing problem of viral resistance in long-term management of HIV also become an obvious issue when the very high rates of viral reproduction were revealed.
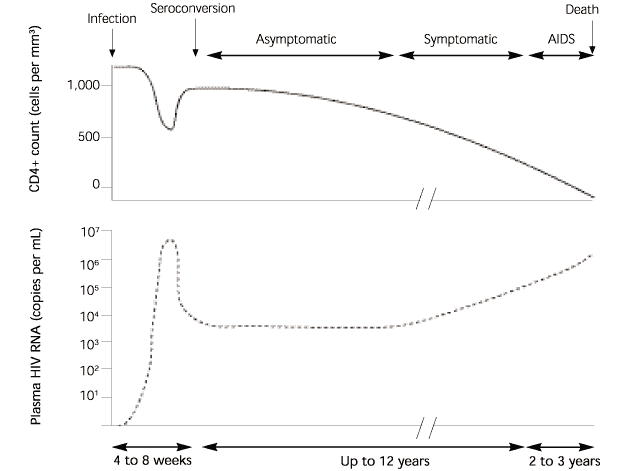
Measurements of plasma viral load (PVL) are now being used routinely in clinical practice (Table 1).6–9 The “baseline” PVL (i.e., the PVL titer taken a few months after serocon-version) is an important predictor of disease progression10,11 (Figure 1).12 Serial measurements of PVL help patients and physicians decide when to begin antiretroviral drug therapy, assist in establishing the effectiveness or failure of therapy, and help ascertain when the beneficial effect of treatment is being lost and therapy must be changed.13
| History and symptoms consistent with acute HIV syndrome |
| Indeterminate HIV antibody test in a patient at high risk for HIV infection |
| Initial evaluation of newly diagnosed HIV infection |
| Surveillance of patients who are not receiving antiretroviral drug therapy |
| Before initiation or change of antiretroviral drug therapy |
| Monthly after initiation of antiretroviral drug therapy and every 1 to 3 months until therapeutic goal is attained* |
PVL Assays
Of the three assays presently available to quantitate PVL (PCR, bDNA and NASBA), the PCR assay requires less plasma. However, the bDNA assay is technically easier to perform, and its results show less variation from laboratory to laboratory.14
Initially, the limit of sensitivity for the three assays was between 200 and 500 HIV RNA copies per mL of plasma, with an upper detection limit of 100,000 to more than 1 million copies per mL.15 More sensitive versions of these assays, with a detection threshold of 20 to 50 copies per mL, are now available.2
A recent evaluation found that the three commercially available tests all had a specificity of 100 percent.16 Furthermore, little difference was found between HIV RNA concentrations and estimates of HIV RNA measured in the different laboratories that participated in the study. However, physicians should be cautious in extrapolating the results of a clinical trial to everyday clinical practice. Comparisons of the results obtained with PCR and bDNA assays consistently indicate that the PVL values obtained by PCR assay are higher than those obtained by the bDNA assay.10 Hence, when feasible, the same assay should be used for serial PVL testing in the individual patient.10
Clinical Use of PVL Testing
A number of studies have compared the prognostic values of PVL testing and other traditional markers of risk for acquired immunodeficiency syndrome (AIDS). A study conducted in a large group of HIV-infected men found that PVL was the single best predictor of clinical outcome, followed (in order of predictive value) by CD4+ T-lymphocyte counts, neopterin levels, β2-microglobulin levels, and thrush or fever.11 A similar study in HIV-infected women also demonstrated an association between PVL and disease prognosis.17
Other studies concluded that the combination of PVL and CD4+ cell counts provided more prognostic information than either factor alone.18,19 These investigations also confirmed the ability of baseline PVL and CD4+ cell counts independently to predict clinical outcome and noted that after the initiation of antiretroviral drug therapy, changes in these markers can predict outcome. Each 0.5-log reduction in PVL has been associated with a 30 percent reduction in the risk of clinical progression, whereas each 10 percent increase in CD4+ cell count has been associated with a 15 percent reduction in risk.10 Moreover, at least in pregnant HIV-infected women, the PVL predicts transmission risk.20
All of the widely used guidelines for the management of HIV-infected patients have incorporated PVL testing for staging disease and determining prognosis.
STARTING ANTIRETROVIRAL DRUG THERAPY
Multiple analyses in more than 5,000 patients who participated in approximately 18 antiretroviral drug trials have shown a significant association between a decrease in PVL and improved clinical outcome.6 Therefore, the U.S. Department of Health and Human Services and the Henry J. Kaiser Foundation,6 as well as the International AIDS Society–USA Panel,7 currently suggest that the results of PVL testing should be an essential parameter in decisions on initiating or changing antiretroviral drug therapy. Measurements of PVL and CD4+ cell count should be performed periodically throughout the course of HIV infection (Table 1).6–9
Given the inherent variability in PVL assays, testing should be performed on at least two separate samples, using the same type of assay and preferably the same laboratory, before treatment decisions are made. Because recent illness or vaccination can lead to transient changes in PVL and CD4+ cell count, assays should be avoided at such times.
The major guidelines vary slightly in the PVL and CD4+ cell cutoff values that are used for recommendations on starting, considering or deferring antiretroviral drug therapy (Table 2).6,7 PVL measurements ranging from 10,000 to 30,000 copies per mL and CD4+ cell counts of less than 350 to 500 per mm3 (0.35 to 0.50 × 109 per L) are cited as indications of the need to initiate antiretroviral drug therapy in most patients.
| Treatment | U.S. Department of Health and Human Services/Henry J. Kaiser Foundation | International AIDS Society–USA Panel |
|---|---|---|
| Start | PVL: > 10,000 to 20,000 copies per mL | PVL: > 30,000 copies per mL |
| CD4+ cell count: < 500 per mm3 (0.50 × 109 per L) | CD4+ cell count: < 350 per mm3 (0.35 × 109 per L) | |
| Consider | NR | PVL: 5,000 to 30,000 copies per mL |
| CD4+ cell count: 350 to 500 per mm3 | ||
| Defer | PVL: < 10,000 copies per mL | PVL: < 5,000 copies per mL |
| CD4+ cell count: > 500 per mm3 | CD4+ cell count: > 500 per mm3 |
Concerns about treatment complexities, adverse effects, possible emergence of viral resistance and limitation of future options are just as important as specific numeric cutoffs in decisions regarding antiretroviral drug therapy. Not all patients will be able to achieve the goal of durable viral suppression, and treatment regimens need to be individualized. The substantial cost, complexity and side effects of long-term therapy require careful attention to the patient's preferences about treatment.
Of note, the viral load appears to be lower in women than in men early in HIV infection, but as immune deficiency advances, gender differences generally disappear.21 Thus, treatment recommendations are the same for women and men.
ASSESSING THE EFFECTIVENESS OF ANTIRETROVIRAL DRUG THERAPY
PVL testing also has a role in optimizing antiretroviral drug therapy. This application has the potential to improve clinical outcomes and decrease the use of antiviral agents that are no longer effective, thereby limiting the emergence of drug-resistant HIV strains. According to current recommendations, the preferred initial antiretroviral drug regimen is one that is most likely to reduce and maintain plasma HIV RNA below the level of detection.6,7
CD4+ cell counts and HIV RNA levels are important tools for evaluating treatment response. As mentioned previously, a minimum of two CD4+ cell counts and PVL measurements should be obtained on separate visits before treatment is changed.22 Ideally, the HIV RNA level should decline rapidly after antiretroviral drug therapy is initiated. Guidelines on the expected PVL reductions vary. A typical goal is a 1- to 2-log reduction within four to eight weeks (e.g., from 50,000 copies per mL to 500 copies per mL).6,7 Failure to achieve the target level of less than 50 copies per mL after 16 to 24 weeks of treatment should prompt consideration of drug resistance, inadequate drug absorption or poor compliance. Maximal viral suppression often takes longer in patients with higher baseline HIV RNA levels (e.g., greater than 100,000 copies per mL). HIV RNA levels should be obtained periodically during antiretroviral drug therapy, although precise data are not available on the optimal frequency of such monitoring (Table 1).6–9
For patients in whom a PVL below detectable level has been achieved, a general guideline is to change antiretroviral drug therapy if the plasma HIV RNA concentration is found to be increasing. Ideally, any confirmed detectable plasma HIV RNA is an indication to change therapy in order to prevent the emergence of drug-resistant viral mutants. In some patients, it may be reasonable to wait to change treatment until there is a documented increase in the plasma HIV RNA level to greater than 2,000 to 5,000 copies per mL. In patients with an initially significant decrease in HIV RNA, (but not to below the detection level), a confirmed increase to greater than 5,000 to 10,000 copies per mL suggests the need for a treatment change.7
Caution should be exercised in interpreting the results of PVL tests. Intra-assay and biologic variability may affect the findings, and concomitant illness or vaccination may cause transient HIV RNA elevations. In addition, all specimens must be processed promptly (ideally, within two to four hours). Because of the rapid pace of viral replication in vivo, patients who miss even a few doses of anti-retroviral drugs before their visit may already be experiencing viral rebound, and their anti-retroviral drug therapy could be incorrectly judged to be failing.4
Before ordering a PVL test, the physician should review the patient's adherence to the antiretroviral drug regimen and should postpone testing if recent doses have been missed. If the HIV RNA level has fallen to near the lower limit of detection by week 24 but is not yet below the detection level, it is not yet clear whether an attempt to change or add to (i.e., intensify) the regimen is indicated. Because lack of adherence to a complete regimen is often the primary reason for treatment failure, alteration of a failing regimen may not directly address the underlying problem.7
Although PVL testing is important, it is not the only factor to consider in evaluating an antiretroviral drug regimen and making decisions on treatment changes. A change in anti-retroviral drug therapy should also be considered if the CD4+ cell count is declining, clinical disease is progressing, medications have unacceptable toxicity or intolerable side effects, or the patient is not adhering to the treatment regimen.7
Numerous clinical guidelines are available to guide physicians and patients through the complicated process of finding the optimal treatment regimen. Skillful selection of initial therapy is important, as the failure of some medications can compromise the subsequent use of other antiretroviral drugs, and a number of medications are more effective when used in specific combinations. The reference list for this article includes several up-to-date sources that can provide guidance in the selection of antiretroviral drug therapy.
PVL Testing in the Initial Diagnosis of HIV Infection
The combination of a screening enzyme-linked immunosorbent assay (ELISA) followed by a confirmatory Western blot test has been more than 99 percent accurate in detecting HIV infection.23 However, this protocol may have negative or indeterminate results, especially during the first weeks of HIV infection24 (Table 3).9,25,26
| Acute seroconversion (usually the first 3 to 4 weeks of HIV infection) |
| Advanced AIDS |
| Autoimmune disease |
| Renal failure and hemodialysis |
| Cystic fibrosis |
| Multiple pregnancies or transfusions |
| Liver disease |
| Injectable drug use |
| Vaccination (hepatitis, rabies, etc.) |
| HIV vaccination (patients participating in clinical trials) |
In most patients with HIV infection, initial viremia occurs within four to 11 days after exposure. During seroconversion, the PVL titer is usually very high.27 The occurrence of these high levels of viremia during primary HIV infection has led some physicians to use PVL assays as diagnostic tests for early HIV infection in high-risk patients with a negative ELISA or an indeterminate Western blot technique.8,9
A drawback to PVL testing is the high cost of the assays. Furthermore, contamination of samples can result in problems,28 and misdiagnosis of HIV infection by PVL testing has been reported.29 To minimize the occurrence of false-positive results, only patients who have a high pretest probability of a positive result should be evaluated for HIV infection using PVL testing.29 Such patients include those with definite or probable recent exposure to HIV and a clinical syndrome suggestive of acute HIV infection.29,30 The typical symptoms of acute HIV infection are similar to those of viral illnesses. Fever, malaise, rash and myalgias are common, as is generalized lymphadenopathy (Table 4).31
| Fever (96 percent) |
| Lymphadenopathy (74 percent) |
| Pharyngitis (70 percent) |
| Rash (70 percent): erythematous maculopapular rash with lesions on the face, trunk and, sometimes, extremities (including palms and soles); mucocutaneous ulcerations involving mouth, esophagus or genitals |
| Myalgia or arthralgia (54 percent) |
| Diarrhea (32 percent) |
| Headache (32 percent) |
| Nausea and vomiting (27 percent) |
| Hepatosplenomegaly (14 percent) |
| Weight loss (13 percent) |
| Thrush (12 percent) |
| Neurologic symptoms (12 percent): meningoencephalitis or aseptic meningitis, peripheral neuropathy or radiculopathy, facial palsy, Guillain-Barré syndrome, brachial neuritis, cognitive impairment or psychosis |
Adapted with permission from Niu MT, Stein DS, Schnittman SM. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in humans and animal retrovirus infections. J Infect Dis 1993;168:1490–501.
Patients with truly acute infection usually have a high PVL, such as 100,000 copies per mL (Figure 1),12 whereas those who have an undetectable or low PVL (a few thousand copies per mL or less) are most likely not infected.8 Even if PVL is detectable, repeat HIV antibody testing is indicated to rule out a false-positive PVL assay.32,33
Final Comment
Because HIV RNA measurements have become part of everyday practice, the family physician should be aware of the strengths and limitations of PVL assays and acquire the necessary experience to optimally use and interpret these tests. When HIV infection is diagnosed, the physician should work with the patient to establish an appropriate target PVL and determine whether and when antiretroviral drug therapy should be initiated.