
A more recent article on diagnosis and treatment of melanoma is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2001;63(7):1359-1369
See patient information handout on malignant melanoma, written by the authors of this article.
The incidence of malignant melanoma has increased in recent years more than that of any other cancer in the United States. About one in 70 people will develop melanoma during their lifetime. Family physicians should be aware that a patient with a changing mole, an atypical mole or multiple nevi is at considerable risk for developing melanoma. Any mole that is suggestive of melanoma requires an excisional biopsy, primarily because prognosis and treatment are based on tumor thickness. Staging is based on tumor thickness (Breslow's measurement) and histologic level of invasion (Clark level). The current recommendations for excisional removal of confirmed melanomas include 1-cm margins for lesions measuring 1.0 mm or less in thickness and 2-cm margins for lesions from 1.0 mm to 4.0 mm in thickness or Clark's level IV of any thickness. No evidence currently shows that wider margins improve survival in patients with lesions more than 4.0 mm thick. Clinically positive nodes are typically managed by completely removing lymph nodes in the area. Elective lymph node dissection is recommended only for patients who are younger than 60 years with lesions between 1.5 mm and 4.0 mm in thickness. In the Eastern Cooperative Oncology Group Trial, interferon alfa-2b was shown to improve disease-free and overall survival, but in many other trials it has not been shown to be effective at prolonging overall survival. Vaccine therapy is currently being used to stimulate the immune system of melanoma patients with metastatic disease.
Malignant melanoma is the eighth most common cancer in the United States and causes 1 to 2 percent of all cancer deaths.1,2 Melanoma is a proliferation of transformed melanocytes or pigment-producing cells. These tumors occur primarily on the skin but may also arise in other tissues where pigment cells are found.
In men, melanomas occur most frequently on the trunk, whereas in women, melanomas occur most frequently on the lower extremities. Melanoma is almost exclusively a disease of adults. In children, melanoma predominantly occurs in the setting of giant congenital nevi or atypical/dysplastic nevus syndrome, or in the setting of xeroderma pigmentosum (an inherited condition of abnormal DNA repair leading to multiple skin cancers at an early age).
Incidence and Impact
The incidence of melanoma has increased in recent years more than that of any other cancer in the United States. In 1999, in the United States, 44,200 people developed invasive melanoma, and 7,300 died from the disease. An additional 30,000 to 50,000 persons developed in situ disease.3 In 1960, one in 1,500 Americans was expected to develop melanoma during their lifetime. In the year 2000, that number was expected to be one in 70.4
Melanoma ranks sixth in cancer incidence in males and seventh in females, and these incidences have doubled in the past decade.2 This increase is not an artifact resulting from improved surveillance techniques, because histologic criteria have remained stable over the decades.4 Because melanoma is not a reportable cancer in all states, and because many cases are treated in an outpatient setting, the true number of melanoma cases may be underreported.5 The mortality rate is increasing by 2 percent per year, while survival rates are improving—which tends to confirm the impression of a true increase in incidence rather than simply an increase in detection.4,6
Risk Factors
| Risk factor | Relative risk* [ corrected] | |
|---|---|---|
| History of a changing mole | >400 | |
| Atypical nevus syndrome | ||
| With a family history of melanoma | 148 | |
| With a personal and family history of melanoma | 500 | |
| Large congenital nevus (15 cm or more in diameter) | 17 | |
| White race | 10 to 12 | |
| Personal history of melanoma | 9 | |
| History of melanoma before age 40 | 23 | |
| Regular tanning bed use before age 30 | 7.7 | |
| Multiple nevi | 5 to 12 | |
| Atypical nevi | 7 to 27 | |
| Immunosuppression | 4 to 8 | |
| Family history (first-degree) of melanoma | 3 to 8 | |
| Nonmelanoma skin cancer | 3 to 5 | |
| Sun sensitivity (tendency to sunburn) | 2 to 3 | |
SKIN TYPE AND SUNBURN HISTORY
People with a white racial background have at least a 10-fold increase in the incidence of melanoma compared with blacks and a sevenfold increased incidence compared with American Hispanics.8 Sun sensitivity refers to a person's tendency to sunburn rather than tan. Persons who have a tendency to burn and freckle rather than tan also have an increased melanoma risk. A history of sunburns, even after the age of 20, is associated with an increased risk of melanoma.9 Blue eyes and red or blonde hair color, although useful as indicators of increased melanoma risk, are not as directly related to increased risk as skin type has been shown to be.10
NONMELANOMA SKIN CANCER
A history of a nonmelanoma (e.g., basal cell or squamous cell) skin cancer may increase a person's risk of developing melanoma by threefold to fivefold.11 This risk is in part compounded when a person has a tendency to sunburn rather than tan and also has a history of cumulative sun exposure.
MULTIPLE NEVI
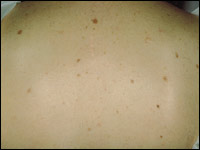
Persons with multiple nevi, particularly nevi that are atypical in appearance, have a significantly increased risk for developing melanoma. Persons with more than 50 common acquired nevi, all of which are greater than 2 mm in diameter, have five to 17 times the risk of developing melanoma than do persons with fewer nevi.10,12 The development of about one melanoma in four is closely associated with a previously existing nevus.13 Thus, patients with multiple benign-appearing nevi must be examined carefully, preferably at least annually, with special attention to any new lesions or changes in existing lesions.
GIANT CONGENITAL NEVI
Giant congenital nevi—those that measure 15 cm in diameter or that are at least twice the size of the palm of an affected person (Figure 1) —have a 6 percent estimated lifetime risk of developing into melanomas. One half of the cases in which melanomas develop in giant nevi occur within the first five years of life. Such melanomas are often quite deep, extending into noncutaneous tissue.14 It is unclear whether medium-sized congenital nevi (1.5 to 15 cm in diameter) have an increased risk of melanoma.15
ATYPICAL/DYSPLASTIC NEVI
Atypical, or dysplastic, nevi (Figures 2a and 2b) are described clinically as intermediate between common nevi and melanoma. Atypical nevi are usually greater than 5 mm in diameter, are irregularly pigmented and have blurred borders and a textured surface. Such nevi may have the morphology of a raised, central papule with a surrounding macular component, thereby giving a “fried-egg” appearance. Not all atypical nevi, however, have the “fried-egg” appearance or textured surface.
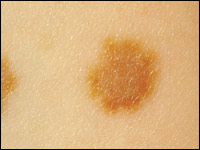
The issue of whether one isolated, slightly atypical or dysplastic nevus is a clinical marker for an increased risk of melanoma is controversial. A patient with only one atypical nevus most likely does not have an increased lifetime risk; but those with multiple atypical nevi (Figure 3) have a relative risk for developing melanoma that may be seven times that of other persons. Patients with multiple atypical nevi, a family history of atypical nevi and two or more family members with melanoma (B-K mole syndrome) have virtually a 100 percent chance of developing a melanoma during their lifetime.16
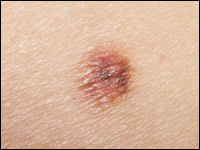
HISTORY OF A CHANGING MOLE
The most predictive features for melanoma development are a history of change in an existing nevus or a new and changing pigmented lesion. The estimated relative risk of the association between melanoma and a changing mole is greater than 400 percent.7 The most important early changes are change in color, diameter increase and border change. Later, when the lesion is more invasive, an increase in height heralds a corresponding growth in depth. Bleeding, ulceration or discomfort are late signs and are associated with a worse prognosis. Because pruritus remains a prevalent early symptom in almost one half of patients with a melanoma, the onset of itching in a new or longstanding mole should not be ignored.
HEREDITY
A family history of melanoma increases a person's risk of developing melanoma by three to eight times compared with persons who have no such history. Persons with familial melanoma D2 (a personal and family history of melanoma in which two or more family members have had melanoma) have virtually a 100 percent lifetime risk of developing melanoma. Approximately 32,000 people in the United States are estimated to be in this familial category.16
TANNING BED USE
The use of a tanning bed 10 times per year or more is linked to a twofold increased risk of melanoma for those older than 30 years. For those younger than 30, this exposure is associated with a risk increased by a factor of 7.7.17
IMMUNOSUPPRESSSION
Immunosuppressed patients—such as those with lymphoma, leukemia or organ transplants—have an increased risk of melanoma.18
Types of Melanomas
Various subtypes of melanomas have been identified: lentigo maligna, superficial spreading, nodular, acral and amelanotic melanomas.
LENTIGO MALIGNA MELANOMAS
Lentigo maligna melanomas (Figure 4) arise in lentigo maligna lesions and represent approximately 5 percent of melanomas. These are clearly related to sun exposure and occur on sun-exposed areas of the skin in adults. Usually, a lapse of many years occurs before a lentigo maligna becomes an invasive melanoma. Vertical growth and metastasis typically occur after many years. Lentigo maligna melanoma was previously thought to behave less aggressively than other melanoma types; however, depth of melanoma, not type, is the most important prognostic factor.
SUPERFICIAL SPREADING MELANOMA
Superficial spreading melanoma is the most common form of melanoma, representing 70 percent of those diagnosed. It can exhibit a prolonged horizontal growth pattern over months to years before becoming invasive (Figure 5).
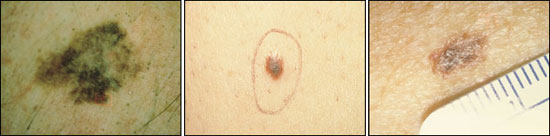
NODULAR MELANOMAS
Nodular melanomas comprise 15 percent of melanomas and may look similar to blood vessel growths (Figure 6). Such melanomas are invasive soon after their appearance, but when controlling for depth of the melanoma, the prognosis is the same as for other lesions.
ACRAL-LENTIGINOUS MELANOMAS
Acral-lentiginous melanomas represent 8 percent of cases of melanoma (Figure 7). They are the most common melanomas in dark-skinned persons. Acral-lentiginous melanomas occur on the palms, soles, nail beds, mucous membranes and penis.
AMELANOTIC MELANOMAS
Amelanotic melanomas (those without pigmentation changes) occur less frequently and are more challenging to diagnose because of the absence of pigmentation. They nonetheless frequently demonstrate changes in size, borders or symmetry.
Diagnosis of Melanoma
WARNING SIGNS AND SYMPTOMS OF MELANOMA
Classically, the “ABCD” mnemonic is used to describe the most common characteristics of a melanoma. These include: moles that are asymmetric, that have irregular borders, that have variability or recent change in color, and that are greater than 6 mm in diameter19 (Table 2). Pruritus, ulceration and bleeding in a mole are warning symptoms. [ corrected] However, clinicians must remember that early melanomas may be less than 6 mm in diameter or may be symmetric, so that any one of the above features can be of concern. The differential diagnosis for melanoma is noted in Table 3.
| A | Asymmetry of mole |
| B | Border irregularity of mole |
| C | Color variability within a lesion |
| D | Diameter increasing, especially to more than 6 mm |
| Condition | Distinguishing characteristics |
|---|---|
| Seborrheic keratosis | “Stuck-on” appearance, symmetric, often multiple |
| Traumatized or irritated nevus | Returns to normal appearance within 7 to 14 days |
| Pigmented basal cell carcinoma | Waxy appearance, telangiectasias |
| Lentigo | Prevalent in sun-exposed skin, evenly pigmented, symmetric |
| Blue nevus | Darkly pigmented from dermal melanocytes, no history of change |
| Angiokeratoma | Vascular tumors, difficult to distinguish from melanoma |
| Traumatic hematoma | May mimic melanoma but resolves in 7 to 14 days |
| Venous lake | Blue, compressible, found on ears and lips |
| Hemangioma | Compressible, stable |
| Dermatofibroma | Firm growths of fibrous histiocytes, “button-hole” when pinched |
| Pigmented actinic keratosis | Sandpapery feel; sun-exposed areas |
BIOPSY
The diagnosis of melanoma is best made on the basis of an excisional biopsy. Any lesion suspected of being a melanoma should be removed completely with a vertical and a horizontal margin. Excision of the entire lesion is recommended at the time of original removal, primarily because prognosis is based on the measurement of the thickness of the tumor from the granular cell layer in the epidermis to the deepest malignant cell (i.e., Breslow's measurement,20 Figure 8). If the lesion is not completely removed, a histopathologic reading of a benign lesion may result. In addition, the appropriateness of lymph node dissection and the need for adjuvant therapy is also affected by the depth of the lesion.
For an excisional biopsy, an elliptical excision should be performed through the subcutaneous fat with 2-mm lateral margins. If an excision is not possible, an incisional biopsy removing the thickest part of the lesion is advised. A 5- to 6-mm punch biopsy into the subcutaneous fat is typically used for this procedure.
PATHOLOGY
The pathologist should be given all salient information regarding the patient and specimen, including any personal or family history of melanoma, the size of the lesion and its morphologic characteristics. Particular care should be taken when the lesions show significant regression (depigmentation), when satellite lesions are present and when the lesion is recurrent or has previously been treated. A recurrent nevus may be interpreted erroneously as a melanoma. When the diagnosis is in question, a reading by a board-certified dermatopathologist should be requested.
A complete pathology report should include tumor thickness (Breslow's measurement), the level of invasion (Clark's level21), growth pattern (nodular, superficial spreading, etc.), margin status, dimensions and presence or absence of ulceration. Based on this information, the tumor is staged22 (Table 4).23 When looking at prognostic indications, Breslow's measurement most closely correlates with survival statistics. However, thin lesions (less than 1 mm) may, rarely, become metastatic. Indicators of high-risk thin lesions (those less than 1 mm thick) include: Clark level IV or level V, ulceration, high mitotic index (greater than six per high power field), vascular or lymphatic invasion, scarcity of melanin in the tumor, location of lesions on the trunk or scalp, and male sex of the patient. The likelihood of thin lesions being high-risk also increases with age.24
Presenting the Diagnosis
When the biopsy report confirms the diagnosis of melanoma, the physician must consider how the diagnosis will be presented to the patient. Giving a diagnosis properly can strengthen the patient-physician relationship, empower the patient to seek appropriate care and even improve outcome.25 A potentially helpful mnemonic for giving the diagnosis is “THREE”: time, hope, repetition, empathy and education. It is critical that the physician schedule a sufficient amount of time to talk with the patient when giving the diagnosis. In addition, the physician must be prepared to offer hope, to repeat key information, to be empathetic and to begin the process of patient education. After the diagnosis is given, the patient should be examined for any other suspicious pigmented lesions, including those of the mucous membranes and the scalp. Lymph nodes should be examined for any enlargement, with emphasis on the primary draining areas of the melanoma. First-degree family members should be notified of their increased risk of melanoma and encouraged to have a skin examination.
Management
EXCISIONAL REMOVAL
Historically, excisions with wide margins (3 to 5 cm) were performed for even thin melanomas. More recent studies have demonstrated that patients undergoing excisions with narrower margins have the same recurrence rates as those with wider margins. The Melanoma Trial of the World Health Organization26 demonstrated that narrower margins do not affect rates of recurrence or survival rates. The current recommendations are 1-cm margins for lesions 1 mm in thickness or less and 2-cm margins for lesions of intermediate thickness (1 mm to 4 mm) or Clark's level IV of any thickness. Currently, no evidence exists to show that wider margins improve survival in patients with lesions more than 4 mm in thickness.
If local recurrence develops, long-term, disease-free survival rates are only 30 percent. Recurrence is believed to correlate more with the biologic potential of the tumor than with procedure failure.22
ADDITIONAL STUDIES
At the time of the initial diagnosis, a chest radiograph is obtained, and a liver panel is obtained, more as a baseline than to detect occult disease. These tests are repeated annually for patients with lesions of greater than 1 mm (Breslow's measurement), and they may be considered for patients with thinner lesions by Breslow's measurement who have a Clark's level of III or greater.27
LYMPH NODE DISSECTION
Clinically positive nodes are typically managed by completely removing the lymph nodes in the area. Elective lymph node dissection is recommended only for use in patients younger than 60 years with lesions between 1.5 and 4 mm in thickness. Data show no improved survival with lymph node dissection in patients with thinner lesions (less than 1.5 mm). Patients with thick tumors (greater than 4 mm) often already have widespread disease; the surgical removal of regional lymph nodes does not positively influence their prognosis.28
Sentinel lymph node biopsy to determine node status microscopically is an additional procedure some clinicians recommend for intermediate tumors (1 to 4 mm thickness) or high-risk thin tumors (Clark's level III or greater, particularly in men with truncal tumors or when ulceration or regression is present) and clinically negative nodes. The degree of involvement of regional lymph nodes is predictive of overall survival and is also a key factor in determining the appropriateness of adjuvant therapy and the patient's eligibility for investigational trials. However, increased survival with sentinel lymph node biopsy has not yet been demonstrated.
Sentinel lymph node biopsy is a two-step procedure that is performed after the initial excision of the melanoma and before the wider excision, because dermal lymphatics are disrupted with the wider excision, and this disruption decreases the accuracy of lymph node detection. The procedure utilizes the concept that the draining nodal basin can be identified along with the sentinel node in the chain that would be most likely to be involved with a metastatic lesion. The node is identified by using lymphoscintigraphy and a radioactive tracer (technetium-labeled sulfur colloid or human serum albumin). A handheld gamma detection probe is used intraoperatively to aid in the identification of the sentinel node. Removal and analysis of this node, using special markers for melanoma (S-100 and HMB 45), is 96 percent predictive of the presence of melanoma metastasis. Patients with evidence of metastatic disease in the sentinel node usually undergo a subsequent full lymph-node dissection within five days.29
Treatments for Metastatic or Advanced Disease
INTERFERON
Adjuvant treatment of melanoma patients with interferon is controversial. Although the Eastern Cooperative Oncology Group Trial EST 168430 demonstrated improvements in both disease-free and overall survival, subsequent studies31 have not shown interferon to have significant benefits in the management of patients with stage II or stage III metastatic melanoma. Currently, the use of adjuvant therapy with interferon alfa-2b (Intron A) is considered controversial in patients with metastatic melanoma.
VACCINE THERAPY
Because melanoma is one of the cancers more closely related to the immune system, much emphasis has been given to immunotherapy and vaccine therapy. Vaccines are being used to stimulate the immune system in patients with metastatic disease.32 Physicians wishing to refer patients with metastatic disease for National Cancer Institute (NCI) sponsored trials should contact the NCI Surgery Branch at 301-496-0997.
OTHER THERAPIES
Distant metastatic lesions may be managed surgically if they are isolated and amenable to an excisional procedure. Melanomas tend not to be radiation-sensitive. Chemotherapeutic regimens are sometimes used in combination with surgical treatments. Hyperthermic therapy and limb perfusion may be used on extremities.33
Following the Patient with a History of Melanoma
Patients with familial melanoma and a personal history of melanoma have a very high risk for the development of another melanoma. These patients should be examined by a dermatologist every three to six months. Baseline high-quality photographs can be helpful in management by aiding the detection of a new lesion or a change in an existing lesion.34 Patients not exhibiting atypical nevus syndrome, with or without a family history of melanoma and thin lesions of less than 1 mm in thickness, should be monitored every six months for two years, then annually. A summary of follow-up regimens is presented in Table 5.27
| Patient criteria | Skin examination/physical examination | Other examinations |
|---|---|---|
| Lesions less than 1 mm in thickness without atypical nevus syndrome and/or family history of melanoma | Every six months for two years, then annually | — |
| Lesions more than 1 mm in thickness and/or Clark's level IV | Every three to six months for three years, then every six to 12 monthsfor two years, then annually | Chest x-ray plus LFTs every six to 12 months; CT scan as clinically indicated |
| Clark's level III or IV every | Every three to six months for three years, then every four to 12 months for two years, then annually | Chest x-ray, LFTs and CBC three to 12 months; CT scan as clinically indicated |
Most recurrences manifest during the first five years after the initial occurrence; later occurrences are possible but rare. Patients with a history of melanoma should be educated about the performance of monthly skin self-examinations, checking for recurrences, new lesions or lymph node involvement. Patients with no recurrence after five years have a less than 5 percent chance of having a second melanoma.27
Melanoma and Pregnancy
Until recently, women with a history of melanoma were discouraged by physicians from becoming pregnant. Currently, women with a history of melanoma are counseled to avoid pregnancy for two years after diagnosis, because that is the period during which the risk of recurrence is greatest. [ corrected] Physicians have now become less dogmatic about counseling the avoidance of pregnancy and discouraging the use of oral contraceptive agents, particularly in patients with thin melanomas. Pregnancy avoidance by patients with thicker lesions and a high risk of recurrence is more appropriate given the problems presented by metastatic disease management during pregnancy or even the risk of metastatic lesions to the fetus.35