
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2001;63(10):1991-1999
See patient information handout on PCV for young children, adapted from one published by the Centers for Disease Control and Prevention.
Streptococcus pneumoniae causes approximately 3,300 cases of meningitis, 100,000 to 135,000 cases of pneumonia requiring hospitalization and 6 million cases of otitis media annually in the United States. Pneumococcal conjugate vaccine, approved in 2000 for use in the United States, was designed to cover the seven serotypes that account for about 80 percent of invasive infections in children younger than six years. This vaccine demonstrated 100 percent efficacy against invasive pneumococcal disease in the primary analysis of a large randomized, double-blind, controlled trial. In the follow-up analysis, performed eight months after the trial ended, efficacy against invasive disease was found to be 94 percent for the included serotypes. When initiated during infancy, the four-dose vaccination schedule is set at two, four, six and 12 to 15 months of age. The American Academy of Family Physicians recommends routine vaccination of infants, catch-up vaccination of children younger than 24 months and catch-up vaccination of children 24 to 59 months of age with high-risk medical conditions such as sickle cell disease and congenital heart disease.
Streptococcus pneumoniae causes approximately 3,300 cases of meningitis, 100,000 to 135,000 cases of pneumonia requiring hospitalization and 6 million cases of otitis media per year in the United States.1 Among children younger than five years, S. pneumoniae causes about 17,000 cases of invasive disease and 200 deaths per year.2 Invasive disease includes bacteremia, meningitis and infection in a normally sterile site, excluding the middle ear. S. pneumoniae is the most common bacterial cause of community-acquired pneumonia, sinusitis and acute otitis media in young children.2 Because Haemophilus influenzae type b (Hib) vaccine has been so successful in reducing meningitis, S. pneumoniae has become the leading cause of bacterial meningitis in the United States.3
The importance of immunizations is heightened because of the increased proportion of antibiotic-resistant S. pneumoniae. In 1998, based on cases of invasive pneumococcal disease detected in the surveillance areas identified in the Centers for Disease Control and Prevention Active Bacterial Core Surveillance Report,1 fully 24 percent of isolates had intermediate susceptibility or were found to be resistant to penicillin. Some isolates have shown resistance to multiple antibiotics.4,5
Streptococcus pneumoniae
S. pneumoniae is a gram-positive diplococci with a polysaccharide capsule that helps protect it from host defense mechanisms. Ninety capsular serotypes have been identified.
Colonization of the nasopharynx with S. pneumoniae is common in healthy persons, and colonized persons are typically asymptomatic. The percentage of persons colonized is about 5 to 10 percent of healthy adults and 20 to 40 percent of healthy children when using a single specimen. The incidence of colonization rises to 40 to 60 percent in toddlers in day care when using repeat specimens.6 Infection is spread by droplets from respiratory tract secretions.
A person's risk for pneumococcal disease is influenced by several factors. First, the normal removal of bacteria by cilia may be interrupted by edema, ciliary damage or increased mucus caused by viral infections or smoking.6 Thus, it is common for viral respiratory tract infections to precede pneumococcal disease, which occurs more commonly during the winter and spring.
Second, in contrast to long-term colonization, infection usually occurs within one month of acquiring a new serotype.7 A third factor is decreased host immunity, including decreased antibody formation such as that occurring in human immunodeficiency virus (HIV) infection, and decreased clearance of pneumococci from the blood stream such as that occurring in asplenia and sickle cell disease.6 A fourth factor is the lack of humoral immunity to a specific serotype. Passive antibodies transferred across the placenta during pregnancy provide protection for neonates, but this protection is lost fairly soon.
Risk Factors for Invasive Disease
Risk factors for invasive pneumococcal disease include age, race, recent use of antibiotics, attendance at group day care, passive exposure to tobacco smoke and chronic medical conditions.5,8,9 Breast-feeding has been shown to have protective qualities. The incidence rates are highest during infancy, decline through the teenage years and then increase in the elderly (Table 1).1,2
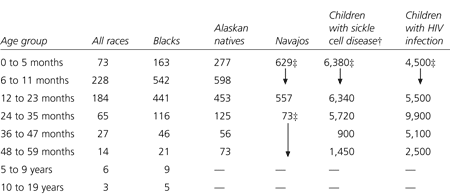
[ corrected] Rates of invasive pneumococcal disease among blacks are about twofold to threefold higher than rates in whites. Rates among Alaskan natives and Native Americans are about threefold to sevenfold higher than rates in whites. The higher rates of disease in these groups may result from underlying factors such as sickle cell disease and poverty. Children with sickle cell disease have high rates of invasive pneumococcal disease. Penicillin prophylaxis has been shown to reduce the risk of pneumococcal disease in these children, but rates are still elevated at about 1,350 per 100,000 children.2
Other predisposing risk factors include other sickle hemoglobinopathies, functional or anatomic asplenia and HIV infection. Attendance at group day care increases a child's risk of contracting invasive pneumococcal disease by twofold to threefold and also increases the risk of developing resistance to penicillin.8
Immunologic Differences in Vaccines
pneumococcus are currently available: the older 23-valent polysaccharide vaccine (PPV; Pnu-Imune 23) and the 7-valent pneumococcal conjugate vaccine (PCV; Prevnar), which was approved for use in 2000.
The polysaccharide vaccine contains T-cell-independent antigens that stimulate mature B-lymphocytes to produce effective antibody but not T-lymphocytes. Thus, T-cell-independent immune responses do not produce an anamnestic response on challenge and may not be long lasting. This vaccine is effective in older children and adults but not in children younger than two years because they do not respond well to these types of antigens. In fact, the serotypes that cause most cases of disease—6A, 14, 19F and 23F—do not induce a good immune response to polysaccharide vaccine until a child is five years of age.10 Finally, the polysaccharide vaccine does not reduce nasopharyngeal colonization of S. pneumoniae, the importance of this finding is debated.
PCV is immunogenic.11,12 The carrier protein is CRM-197, which has been used in one Hib vaccine. PCV does not contain thimerosal. The vaccine was designed to cover the seven serotypes (4,6B, 9V, 14,18C, 19F and 23F) that are found most commonly in children. These serotypes account for about 80 percent of invasive infections in children younger than six years but only 50 percent of infections in those six years and older13 (Figure 1).1
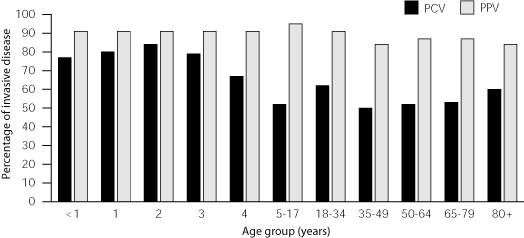
Obviously, PCV covers fewer serotypes than the polysaccharide vaccine; however, with regard to the serotypes in PCV, the latter is more immunogenic than the polysaccharide vaccine. PCV elicits a T-cell-dependent immune response that leads to an anamnestic response on challenge and is effective in infants. PCV reduces nasopharyngeal carriage of S. pneumoniae and could theoretically create herd immunity based on experience with Hib vaccine. The incidence of Hib disease declined in infants after the Hib vaccine was used in toddlers and before it was approved for use in infants.
Efficacy of PCV
In the interim efficacy analysis of a randomized, double-blind, controlled trial, efficacy of PCV was shown to be 100 percent. In the follow-up analysis14 performed eight months after the trial ended, the vaccine's efficacy against invasive disease was shown to be 94 percent for serotypes included in the vaccine in the intent-to-treat (ITT) analysis and 97 percent for serotypes in the vaccine among persons who were fully vaccinated. The vaccine's efficacy against all serotypes, including nonvaccine types, was 89 percent, suggesting some cross-protection among related sero-types.14 In the ITT analysis, efficacy was 11 percent against clinical pneumonia, 33 percent against clinical pneumonia supported with any radiographic evidence of infiltrate and 73 percent against pneumonia with radiographic evidence of consolidation of 2.5 cm or more. Of course, radiographic evidence of consolidation is more typical of pneumococcal pneumonia, whereas clinically diagnosed pneumonia is often viral.
The efficacy in the ITT analysis was 6.4 percent against otitis media, 10 percent against frequent otitis media (i.e., four or more episodes in six months or five or more episodes in one year) and 20 percent against ventilatory tube placement.14 The vaccine has lower efficacy in otitis media because most cases are caused by other organisms, the serotypes in otitis media differ somewhat from those in the vaccine and protective antibody concentrations are not always achieved in the middle ear. The vaccine also reduced the use of antibiotics by 5.3 percent. The number needed to treat is 411 to prevent an episode of invasive disease, 239 to prevent pneumonia and 151 to prevent invasive disease or pneumonia.
Published analyses show that vaccinating healthy infants would prevent more than 12,000 cases of meningitis and bacteremia, 53,000 cases of pneumonia and 1 million cases of otitis media per year.15 The break-even price of PCV is $46 per dose from the societal perspective and $18 per dose from the health care payer's perspective.15 The manufacturer's list price is about $58 per dose,15 which makes it the most expensive routine infant immunization series to date. At this price, infant vaccination would cost $80,000 per year of life saved and $3,200 per pneumonia case prevented.
Actual prices vary by setting. The federal bulk purchase price through the Vaccines for Children Program is about $46 per dose (personal communication with Dean D. Mason, Chief, Program Support Branch, Immunization Services Division, National Immunization Program, Centers for Disease Control and Prevention, November 2000).
Adverse Reactions
No serious adverse reactions are associated with PCV. [ corrected] When administered at the same time as diphtheria and tetanus toxoids and acellular pertussis vaccine (DtaP) at a separate site, a fever of 38°C (100°F) or higher occurred in 15 to 24 percent of children vaccinated with PCV compared with 9 to 17 percent of those receiving the control vaccine (experimental meningococcal conjugate vaccine).14 Among children who received PCV, 10 to 14 percent developed redness at the injection site, and 15 to 23 percent developed tenderness at the injection site. Fever higher than 39°C (102°F) was uncommon, occurring in 1 to 2.5 percent of children who received the vaccination.14
Contraindications and Precautions
The contraindication to PCV is hypersensitivity (e.g., anaphylaxis) to a previous dose or to any component of the vaccine. The vaccine's safety during pregnancy has not been evaluated. Simultaneous administration of PCV and PPV is not recommended because the safety has not been evaluated.
Administration
PCV can be administered at the same time as other childhood vaccines, at a separate site. Studies of interference have found mild suppression of the response to Hib vaccine after the fourth dose; however, more than 97 percent of infants showed seroprotection to H. influenzae type b when that vaccine was administered with PCV.
PCV should be stored refrigerated at 2°C (36°F) to 8°C (46°F). It is administered intramuscularly as a 0.5-mL dose. The CPT code is 90669.
According to U.S. law, a vaccine information sheet must be given to a parent or guardian before administration of routine childhood vaccines, including PCV (see patient education handout or visitwww.cdc.gov/nip).
Recommendations
After PCV was approved by the U.S. Food and Drug Administration, three organizations made recommendations for its use. They are the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention, the American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP) (Table 2).2,16,17
| Age and risk factors | ACIP | AAP | AAFP* |
|---|---|---|---|
| Routine infant vaccination and catch-up for children younger than 23 months | Recommended | Recommended | Recommended as standard |
| Catch-up for children 24 to 59 months of age with high-risk conditions | Recommended | Recommended for those with rates > 150 per 100,000 | Recommended as standard |
| Catch-up for healthy children 24 to 35 months of age | Should be considered a priority | Consider as moderate risk; inadequate data to routinely recommend | Not addressed |
| Catch-up for healthy children of Alaskan native, Native American or black descent, 24 to 59 months of age | Should be considered a priority | Consider as moderate risk; inadequate data to routinely recommend | Recommended |
| Catch-up for healthy children 24 to 59 months of age in group day care | Should be considered a priority | Consider as moderate risk; inadequate data to routinely recommend | Practice option |
| Catch-up for healthy children 36 to 59 months of age | Should be considered | May be elected | Not addressed |
| Catch-up for children 24 to 59 months of age with frequent otitis media | Not addressed | May be considered | Practice option |
| Catch-up for children five years and older | Not contraindicated | May be elected | Not addressed |
Each of these organizations recommends PCV for routine infant immunization and catch-up vaccination of children 23 months and younger. There are three reasons for establishing 23 months of age as the upper limit for routine catch-up vaccination of all healthy children: (1) the incidence rate of invasive disease drops substantially by 24 months of age (Table 1)2; (2) by two years of age, children's immune systems are more mature, and they are better able to withstand pneumococcal infection than younger children; and (3) the cost of the vaccine is substantial when compared to other childhood immunization series and, considering that resources are limited in some situations, the priority should be to vaccinate children who are at highest risk.
The ACIP, AAP and AAFP all recommend catch-up vaccination of children 24 to 59 months of age with high-risk conditions (Table 2).2,16,17 These conditions include sickle cell disease, asplenia, HIV infection, chronic illness (e.g., bronchopulmonary dysplasia, congenital heart disease, congestive heart failure, diabetes mellitus and cerebrospinal fluid leaks), immunocompromising conditions, including congenital immune or complement deficiencies, renal failure, nephrotic syndrome, malignancies and treatment with immune suppressive or radiation therapy (e.g., solid organ transplantation). Penicillin prophylaxis should be continued in children with sickle cell disease after vaccination with PCV.
Recommendations for catch-up vaccination of healthy children 24 to 59 months of age vary by organization (Table 2).2,16,17 The incidence rates of disease decrease with increasing age during childhood, and the case can be made for establishing different cut-off points. Individual risk factors may help when considering which recommendation to follow. (As previously mentioned, risk factors for invasive pneumococcal disease include attendance at group day care, passive exposure to tobacco smoke and race.) In accordance with the increased risk data for race (Table 1)2 and advice from the National Medical Association, the AAFP recommends catch-up vaccination of children 24 to 59 months of age who are of Alaskan native, American Indian and black descent. The ACIP and AAP recommendations state that vaccination can be considered for children in these groups (Table 2).2,16,17
PCV is not approved for use in adults, and no efficacy data are available for its use in older children and adults. Because serotypes change with age, only 50 percent of the serotypes that cause infection in older children and adults are covered by PCV compared with 80 to 90 percent coverage with PPV (Figure 1).1,13 Although ACIP and AAP recommendations allow the use of PCV in older children who have high-risk conditions, PCV should not replace polysaccharide vaccine in older children or adults.
Vaccination Schedule
In infants, the routine vaccine schedule is two, four, six and 12 to 15 months. Table 32 shows a catch-up vaccination schedule. The number of doses varies with age and the presence of high-risk medical conditions. A schedule has been devised for use in children when a lapse in immunization has occurred (Table 4).2 Children at highest risk for complications from pneumococcus may benefit from receiving the polysaccharide vaccine after they have been vaccinated with PCV (Table 5).2
| Age at first dose | Primary series | Booster dose | |
|---|---|---|---|
| 2 to 6 months | Three doses, two months apart* | One dose at 12 to 15 months† | |
| 7 to 11 months | Two doses, two months apart* | One dose at 12 to 15 months† | |
| 12 to 23 months | Two doses, two months apart | — | |
| 24 to 59 months | |||
| Healthy children | One dose | — | |
| Children with sickle cell disease, asplenia, HIV infection, chronic illness or immunocompromising condition‡ | Two doses, two months apart | — | |
| Age at presentation (months) | Previous PCV immunization history | Recommended regimen |
|---|---|---|
| 7 to 11 | One dose | One dose at 7 to 11 months followed by a booster at 12 to 15 months with a minimal interval of 2 months |
| Two doses | One dose at 7 to 11 months followed by a booster at 12 to 15 months with a minimal interval of 2 months | |
| 12 to 23 | One dose before 12 months | Two doses at least 2 months apart |
| Two doses before 12 months | One dose at least 2 months following the most recent dose | |
| 24 to 59 | Any incomplete schedule | One dose* |
| Health status | PPV schedule | Revaccinate with PPV |
|---|---|---|
| Healthy | None | No |
| Sickle cell disease, anatomic or functional asplenia, HIV-infection, immunocompromising conditions | 1 dose PPV given at least 2 months after PCV | Yes* |
| Chronic illness | 1 dose PPV given at least 2 months after PCV | No |