
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2001;63(12):2413-2421
Acute osteomyelitis is the clinical term for a new infection in bone. This infection occurs predominantly in children and is often seeded hematogenously. In adults, osteomyelitis is usually a subacute or chronic infection that develops secondary to an open injury to bone and surrounding soft tissue. The specific organism isolated in bacterial osteomyelitis is often associated with the age of the patient or a common clinical scenario (i.e., trauma or recent surgery). Staphylococcus aureus is implicated in most patients with acute hematogenous osteomyelitis. Staphylococcus epidermidis, S. aureus, Pseudomonas aeruginosa, Serratia marcescens and Escherichia coli are commonly isolated in patients with chronic osteomyelitis. For optimal results, antibiotic therapy must be started early, with antimicrobial agents administered parenterally for at least four to six weeks. Treatment generally involves evaluation, staging, determination of microbial etiology and susceptibilities, antimicrobial therapy and, if necessary, debridement, dead-space management and stabilization of bone.
Osteomyelitis is an inflammation of bone caused by a pyogenic organism. Historically, osteomyelitis has been categorized as acute, subacute or chronic, with the presentation of each type based on the time of disease onset (i.e., occurrence of infection or injury). Acute osteomyelitis develops within two weeks after disease onset, subacute osteomyelitis within one to several months and chronic osteomyelitis after a few months.
Because osteomyelitis is a complex disease state, various classification systems have emerged beyond the general categories of acute, subacute and chronic. The Waldvogel classification system1–3 divides osteomyelitis into the categories of hematogenous, contiguous and chronic (Table 1).1 The more recent Cierny-Mader staging system is based on the status of the disease process, not etiology, chronicity or other factors (Table 2).4 The terms “acute” and “chronic” are not used in the Cierny-Mader system. The stages in this system are dynamic and may be altered by changes in the medical condition of the patient (host), successful antibiotic therapy and other treatments.
| Hematogenous osteomyelitis | |
| Osteomyelitis secondary to contiguous focus of infection | |
| No generalized vascular disease | |
| Generalized vascular disease | |
| Chronic osteomyelitis (necrotic bone) | |
| Anatomic type | |
| Stage 1: medullary osteomyelitis | |
| Stage 2: superficial osteomyelitis | |
| Stage 3: localized osteomyelitis | |
| Stage 4: diffuse osteomyelitis | |
| Physiologic class | |
| A host: healthy | |
| B host: | |
| Bs: systemic compromise | |
| Bl: local compromise | |
| Bls: local and systemic compromise | |
| C host: treatment worse than the disease | |
| Factors affecting immune surveillance, metabolism and local vascularity | |
| Systemic factors (Bs): malnutrition, renal or hepatic failure, diabetes mellitus, chronic hypoxia, immune disease, extremes of age, immunosuppression or immune deficiency | |
| Local factors (Bl): chronic lymphedema, venous stasis, major vessel compromise, arteritis, extensive scarring, radiation fibrosis, small-vessel disease, neuropathy, tobacco abuse | |
Although the classification systems for osteomyelitis help describe the infection and determine the need for surgery, the categories do not apply to special circumstances (i.e., infections involving prosthetic joints, implanted materials or smaller bones of the body) or special types of infection (e.g., vertebral osteomyelitis).
Clinical Description
Acute hematogenous osteomyelitis occurs predominantly in children, with the metaphysis of long bones the most common location. Patients usually present within several days to one week after the onset of symptoms. In addition to local signs of inflammation and infection, patients have signs of systemic illness, including fever, irritability and lethargy. Typical clinical findings include tenderness over the involved bone and decreased range of motion in adjacent joints. The diagnosis of acute osteomyelitis can be established based on several specific clinical findings (Table 3).5
| Pus on aspiration |
| Positive bacterial culture from bone or blood |
| Presence of classic signs and symptoms of acute osteomyelitis |
| Radiographic changes typical of osteomyelitis |
The subacute and chronic forms of osteomyelitis usually occur in adults. Generally, these bone infections are secondary to an open wound, most often an open injury to bone and surrounding soft tissue. Localized bone pain, erythema and drainage around the affected area are frequently present. The cardinal signs of subacute and chronic osteomyelitis include draining sinus tracts, deformity, instability and local signs of impaired vascularity, range of motion and neurologic status. The incidence of deep musculoskeletal infection from open fractures has been reported to be as high as 23 percent.6 Patient factors, such as altered neutrophil defense, humoral immunity and cell-mediated immunity, can increase the risk of osteomyelitis.
Diagnosis
The diagnosis of osteomyelitis is based primarily on the clinical findings, with data from the initial history, physical examination and laboratory tests serving primarily as benchmarks against which treatment response is measured. Leukocytosis and elevations in the erythrocyte sedimentation rate and C-reactive protein level may be noted. Blood cultures are positive in up to one half of children with acute osteomyelitis.
The palpation of bone in the depths of infected pedal ulcers in patients with diabetes mellitus is strongly correlated with the presence of underlying osteomyelitis (sensitivity, 66 percent; specificity, 85 percent; positive predictive value, 89 percent; negative predictive value, 56 percent).7 If bone is palpated, the evaluation may proceed directly to microbiologic and histologic confirmation of osteomyelitis, and thereafter to treatment. Further diagnostic studies are unnecessary.
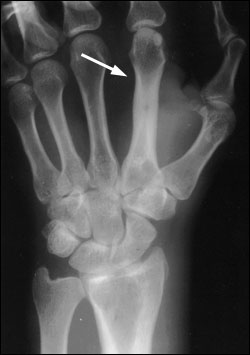
In osteomyelitis of the extremities, plainfilm radiography and bone scintigraphy remain the primary investigative tools8,9 (Table 4).9 Radiographic evidence of bone destruction by osteomyelitis may not appear until approximately two weeks after the onset of infection (Figure 1). The radiographs may reveal osteolysis, periosteal reaction and sequestra (segments of necrotic bone separated from living bone by granulation tissue).10 A bone abscess found during the subacute or chronic stage of hematogenous osteomyelitis is known as a Brodie's abscess.
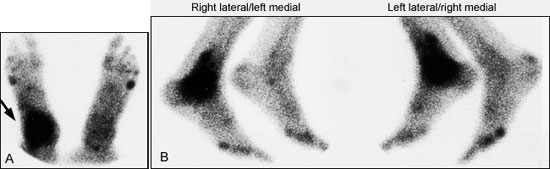
For nuclear imaging, technetium Tc-99m methylene diphosphonate is the radiopharmaceutical agent of choice11 (Figure 2). The specificity of bone scintigraphy will not be high enough to confirm the diagnosis of osteomyelitis in many clinical situations.12 On a bone scan, osteomyelitis often cannot be distinguished from a soft tissue infection, a neurotrophic lesion, gout, degenerative joint disease, postsurgical changes, a healing fracture, a noninfectious inflammatory reaction or a stress fracture. In many instances, a bone scan will be positive despite the absence of bone or joint abnormality.
Magnetic resonance imaging (MRI) can be extremely helpful in unclear situations This imaging modality is particularly useful when a patient is suspected of having osteomyelitis, discitis or septic arthritis involving the axial skeleton and pelvis. Compared with bone scintigraphy, MRI has equivalent or greater sensitivity, specificity and accuracy for the detection of osteomyelitis. MRI also provides greater spatial resolution in delineating the anatomic extension of infection.13
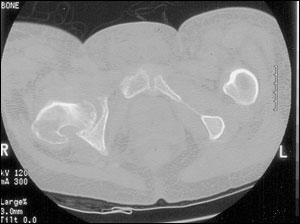
Ultrasonography and computed tomographic (CT) scanning (Figure 3) may be helpful in the evaluation of suspected osteomyelitis.10 An ultrasound examination can detect fluid collections (e.g., an abscess) and surface abnormalities of bone (e.g., periostitis), whereas the CT scan can reveal small areas of osteolysis in cortical bone, small foci of gas and minute foreign bodies.
Histopathologic and microbiologic examination of bone is the gold standard for diagnosing osteomyelitis. Cultures of sinus tract samples are not reliable for identifying causative organisms. Therefore, biopsy is advocated to determine the etiology of osteomyelitis.14 However, the accuracy of biopsy is often limited by lack of uniform specimen collection and previous antibiotic use.
In the evaluation of suspected osteomyelitis, other diagnoses should also be considered. Acute leukemia, cellulitis and malignant bone tumors (i.e., Ewing's sarcoma, osteosarcoma) are conditions with similar presentations.
Etiology
The specific microorganism(s) isolated from patients with bacterial osteomyelitis is often associated with the age of the patient or the clinical scenario (Tables 515 and 616). Staphylococcus aureus is implicated in most cases of acute hematogenous osteomyelitis and is responsible for up to 90 percent of cases in otherwise healthy children.17 Staphylococcus epidermidis, S. aureus, Pseudomonas aeruginosa, Serratia marcescens and Escherichia coli are commonly isolated in patients with chronic osteomyelitis.
| Infants (< 1 year) |
| Group B streptococci |
| Staphylococcus aureus |
| Escherichia coli |
| Children (1 to 16 years) |
| S. aureus |
| Streptococcus pyogenes |
| Haemophilus influenzae |
| Adults (> 16 years) |
| Staphylococcus epidermidis |
| S. aureus |
| Pseudomonas aeruginosa |
| Serratia marcescens |
| E. coli |
| Organism | Comments |
|---|---|
| Staphylococcus aureus | Organism most often isolated in all types of osteomyelitis |
| Coagulase-negative staphylococci or Propionibacterium species | Foreign-body–associated infection |
| Enterobacteriaceae species or Pseudomonas aeruginosa | Common in nosocomial infections |
| Streptococci or anaerobic bacteria | Associated with bites, fist injuries caused by contact with another person's mouth, diabetic foot lesions, decubitus ulcers |
| Salmonella species or Streptococcus pneumoniae | Sickle cell disease |
| Bartonella henselae | Human immunodeficiency virus infection |
| Pasteurella multocida or Eikenella corrodens | Human or animal bites |
| Aspergillus species, Mycobacterium avium-intracellulare or Candida albicans | Immunocompromised patients |
| Mycobacterium tuberculosis | Populations in which tuberculosis is prevalent |
| Brucella species, Coxiella burnetii (cause of chronic Q fever) or other fungi found in specific geographic areas | Population in which these pathogens are endemic |
Treatment
After the initial evaluation, staging and establishment of microbial etiology and susceptibilities, treatment includes antimicrobial therapy, debridement with management of resultant dead space and, if necessary, stabilization of bone.18 In most patients with osteomyelitis, early antibiotic therapy produces the best results. Antimicrobials must be administered for a minimum of four weeks (ideally, six weeks) to achieve an acceptable rate of cure (Table 7).16,19 To reduce costs, parenteral antibiotic administration on an outpatient basis or the use of oral antibiotics can be considered.
| Organism | Antibiotic(s) of first choice | Alternative antibiotics |
|---|---|---|
| Staphylococcus aureus or coagulasenegative (methicillin-sensitive) Staphylococci | Nafcillin (Unipen), 2 g IV every 6 hours, or clindamycin phosphate (Cleocin Phosphate), 900 mg IV every 8 hours | First-generation cephalosporin or vancomycin (Vancocin) |
| S. aureus or coagulase-negative (methicillin-resistant) staphylococci | Vancomycin, 1 g IV every 12 hours | Teicoplanin (Targocid),* trimethoprim- sulfamethoxazole (Bactrim, Septra) or minocycline (Minocin) plus rifampin (Rifadin) |
| Various streptococci (groups A and B β-hemolytic organisms or penicillin-sensitive Streptococcus pneumoniae) | Penicillin G, 4 million units IV every 6 hours | Clindamycin, erythromycin, vancomycin or ceftriaxone (Rocephin) |
| Intermediate penicillin-resistant S. pneumoniae | Cefotaxime (Claforan), 1 g IV every 6 hours, or ceftriaxone, 2 g IV once daily | Erythromycin or clindamycin |
| Penicillin-resistant S. pneumoniae | Vancomycin, 1 g IV every 12 hours | Levofloxacin (Levaquin) |
| Enterococcus species | Ampicillin, 1 g IV every 6 hours, or vancomycin, 1 g IV every 12 hours | Ampicillin-sulbactam (Unasyn) |
| Enteric gram-negative rods | Fluoroquinolone (e.g., ciprofloxacin [Cipro], 750 mg orally every 12 hours) | Third-generation cephalosporin |
| Serratia species or Pseudomonas aeruginosa | Ceftazidime (Fortaz), 2 g IV every 8 hours (with an aminoglycoside given IV once daily or in multiple doses for at least the first 2 weeks) | Imipenem (Primaxin I.V.), piperacillin-tazobactam (Zosyn) or cefepime (Maxipime; given with an aminoglycoside) |
| Anaerobes | Clindamycin, 600 mg IV or orally every 6 hours | For gram-negative anaerobes: amoxicillin-clavulanate (Augmentin) or metronidazole (Flagyl) |
| Mixed aerobic and anaerobic Organisms | Amoxicillin-clavulanate, 875 mg and 125 mg, respectively, orally every 12 hours | Imipenem |
Very few studies have investigated the treatment of osteomyelitis. One review20 found only five studies involving 154 patients with this bone infection.21–25 Delineation of treatment has been difficult for numerous reasons: debridement obscures the impact of antibiotics, clinical situations and pathogens are heterogenous, and years of follow-up are necessary to demonstrate sustained remission. In addition, many studies have not been randomized, have not had a control group and have enrolled only a small number of patients. Therefore, most recommendations for the treatment of osteomyelitis are based on expert opinion rather than the results of randomized, controlled trials.
ANTIBIOTIC THERAPY
Acute hematogenous osteomyelitis is best managed with careful evaluation of microbial etiology and susceptibilities and a four- to six-week course of appropriate antibiotic therapy.
Surgical debridement is not necessary when the diagnosis of hematogenous osteomyelitis is made early. Current treatment recommendations rarely require surgical debridement. However, if antibiotic therapy fails, debridement (or repeated debridement) and another four- to six-week course of parenteral antibiotic therapy is essential.26–28
After cultures have been obtained, an empiric parenteral antibiotic regimen (nafcillin [Unipen] plus either cefotaxime [Claforan] or ceftriaxone [Rocephin]) is initiated to cover clinically suspected organisms. When the culture results are known, the antibiotic regimen is revised.
Chronic osteomyelitis in adults is more refractory to therapy and is generally treated with antibiotics and surgical debridement. Empiric antibiotic therapy is not usually recommended. Depending on the type of chronic osteomyelitis, patients may be treated with parenteral antibiotics for two to six weeks. However, without adequate debridement, chronic osteomyelitis does not respond to most antibiotic regimens, no matter what the duration of therapy is. Outpatient intravenous therapy using long-term intravenous access catheters (i.e., Hickman catheters) decreases the length of hospital stays.28–30
Oral therapy using fluoroquinolone antibiotics for gram-negative organisms is presently being used in adults with osteomyelitis.23 None of the currently available fluoroquinolones provides optimal antistaphylococcal coverage, an important disadvantage in view of the rising incidence of nosocomially acquired staphylococcal resistance.31 Furthermore, the current quinolones provide essentially no coverage of anaerobic pathogens.
DEBRIDEMENT
Surgical debridement in patients with chronic osteomyelitis can be technically demanding.32 The quality of the debridement is the most critical factor in successful management. After debridement with excision of bone, it is necessary to obliterate the dead space created by the removal of tissue. Dead-space management includes local myoplasty, free-tissue transfers and the use of antibiotic-impregnated beads. Soft tissue procedures have been developed to improve local blood flow and antibiotic delivery.
Special Situations
VERTEBRAL OSTEOMYELITIS
Vertebral osteomyelitis commonly stems from a disc-space infection seeded through hematogenous dissemination or surgery.33 Other possible causes are trauma, extension of infection from adjacent structures and complications of spine and disc surgery. Predis posing conditions include an extraspinal infection site, urinary tract instrumentation, indwelling vascular catheter, hemodialysis, intravenous drug abuse, cancer and diabetes mellitus.34 Vertebral osteomyelitis is usually associated with severe pain and limited ability to function.
MRI is an important imaging modality for detecting pyogenic vertebral osteomyelitis.13 This form of osteomyelitis is usually cured without surgery, even though there may be extensive bone involvement. A six-week course of antibiotic therapy is commonly recommended.
PROSTHETIC JOINT INFECTIONS
Coagulase-negative staphylococci are the most common bacteria in prosthetic joint infections. Intravenously administered antibiotics, in addition to surgical removal of the prosthesis, is the best treatment. The joint is left out while a two- to six-week course of intravenous therapy is given; another joint is then implanted.33 Proposed therapies for prosthetic joint infections include the use of antibiotic-impregnated beads and antibiotic-loaded prostheses.
DIABETES MELLITUS
Diabetes is a significant contributing factor in osteomyelitis, particularly when patients have concomitant neurologic or vascular abnormalities.33 A wide variety of organisms (e.g., P. aeruginosa, staphylococci, anaerobes) are frequently isolated from these infections. Initial hospitalization to assess vascular supply, identify offending microbes, remove dead tissue, drain wounds and assure compliance may be necessary.
Follow-up
Early antibiotic therapy, before extensive destruction of bone, produces the best results in patients with osteomyelitis. During treatment, patients should be followed closely for signs and symptoms of worsening infection. After the completion of treatment, follow-up should be based on the response to therapy and the overall health of the patient.