
A more recent article on end of life care is available.
Am Fam Physician. 2001;64(5):807-815
Physical symptoms other than pain often contribute to suffering near the end of life. In addition to pain, the most common symptoms in the terminal stages of an illness such as cancer or acquired immunodeficiency syndrome are fatigue, anorexia, cachexia, nausea, vomiting, constipation, delirium and dyspnea. Management involves a diagnostic evaluation for the cause of each symptom when possible, treatment of the identified cause when reasonable, and concomitant treatment of the symptom using nonpharmacologic and adjunctive pharmacologic measures. Part I of this two-part article discusses fatigue, anorexia, cachexia, nausea and vomiting. Fatigue is the most common symptom at the end of life, but little is known about its pathophysiology and specific treatment. Education of the patient and family is the foundation of treatment, with the possible use of adjunctive psychostimulants. Anorexia and cachexia caused by wasting syndromes are best managed with patient and family education, as well as a possible trial of appetite stimulants such as megestrol or dexamethasone. For appropriate pharmacologic treatment, it is helpful to identify the pathophysiologic origin of nausea in each patient.
Although pain is commonly associated with end-of-life distress, other physical symptoms often contribute to the suffering of terminally ill patients.1–5 In patients who have cancer, fatigue and anorexia rank as the top two reasons for emotional and physical distress, with pain ranked third. Nausea, constipation, altered mental state (e.g., delirium) and dyspnea are the next most common symptoms.3,4 This two-part article focuses on the more prevalent nonpain physical symptoms found in patients who have cancer, acquired immunodeficiency syndrome (AIDS) or other terminal illnesses.
In managing a nonpain symptom at the end of life, the physician should search for the cause of the symptom by obtaining the proper historical, physical and laboratory data (to the extent that is appropriate in terminally ill or hospice patients). Efforts are directed at alleviating the symptom, as well as preventing or treating the underlying cause when possible or reasonable. Effective management requires that the physician be thoroughly familiar with the drugs and treatments prescribed. Frequent re-evaluation of the patient is also important.
Treatments often change as the patient approaches the end of life. The continued presence of physicians and a steadfast commitment to ameliorating symptoms are particularly important when it is impossible to cure or even slow the progression of an underlying disease process.
Fatigue
The terms “asthenia” and “fatigue” are often used interchangeably. Used in relation to a terminal illness, however, the word “fatigue” has multiple components, including the symptoms of tiredness, a general lack of energy not relieved by rest, diminished mental capacity and the subjective weakness associated with difficulty in performing activities of daily living.6 When conducting a review of systems, the physician is advised to use terms that are more familiar to patients than “asthenia.” In addition to “fatigue,” appropriate terms include “tiredness” and “lack of energy.”
Fatigue can be extremely debilitating and may have a severe negative impact on quality of life. This symptom is a problem in 75 to 90 percent of patients with cancer or other chronic illnesses.4–7 Even survivors of cancer and other life-threatening illnesses report chronic fatigue lasting months to years after the completion of treatment. Despite the high prevalence of fatigue, little is known about its pathogenesis. Consequently, treatment for fatigue may be less successful than treatment for other symptoms at the end of life.
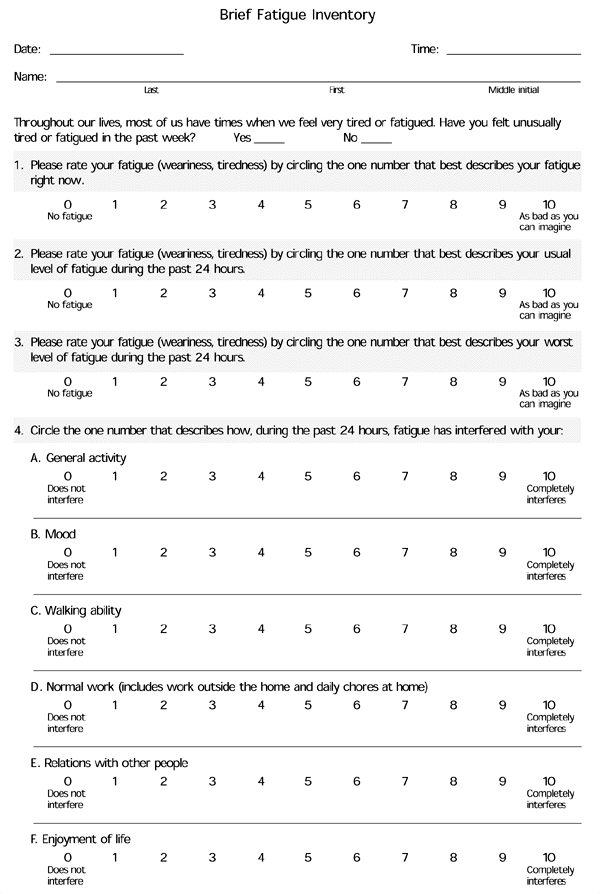
In medical practice, fatigue is frequently undiagnosed or ignored. Recent reports indicate that patients with cancer seldom discuss this symptom with their oncologist.6,8,9 A number of multidimensional fatigue assessment tools have been validated,10–13 but family physicians may be most interested in recently proposed practical criteria for the bedside diagnosis of cancer-related fatigue.6,8 The Brief Fatigue Inventory is a straightforward diagnostic tool (Figure 1).13 (This inventory can also be obtained from the Internet athttp://prg.mdanderson.org/bfi.pdf.)
Pathologic fatigue can arise from both physical and psychologic stresses.6 Physical causes include the direct consequences of a disease process, such as diminished oxygen-carrying capacity as a result of anemia or heart failure. Cancer, hepatic or renal failure, and many chronic illnesses (including chronic pain) can cause fatigue. In addition, treatments such as cancer therapy or anti-hypertensive and cardiac therapy can cause this symptom. Psychologic causes of fatigue include anxiety and depression.
Determining the severity of a patient's fatigue is important. The physician should note the factors that worsen or relieve fatigue, the presence of potentially treatable causes (i.e., anxiety or depression, concurrent medications, anemia, pain, infection, cancer, sleep disorder) and the impact of fatigue on the patient's daily activities and quality of life.6
The management of fatigue with an unidentified underlying treatable cause is summarized in Table 1.6,14 Patient and family education can be of great value. For example, family members may interpret fatigue to mean that the patient is “giving up,” when the symptom is actually beyond the patient's control. To decrease pressure on the patient to be more energetic, the physician may need to give the patient “permission” to rest.14
Medications, notably corticosteroids and psychostimulants, are sometimes beneficial adjuncts to nonpharmacologic interventions directed at relieving fatigue in patients nearing the end of life. Dexamethasone (Decadron), 2 to 20 mg taken orally once daily in the morning, can bring about feelings of well-being and increased energy, although these effects may diminish after the drug has been used for four to six weeks.14 In the end-of-life setting, the long-term side effects of morning doses of corticosteroids are usually not an issue. Of the psychostimulants, methylphenidate (Ritalin) is most commonly prescribed, although dextroamphetamine (Dexedrine) and pemoline (Cylert) also are used.
Antidepressants have been used empirically in patients without clinical depression who have fatigue that does not respond to non-pharmacologic interventions, corticosteroids or psychostimulants. In addition to elevating mood, antidepressants (particularly selective serotonin reuptake inhibitors) can have an energizing effect.6
Erythropoietin therapy can be beneficial in relieving fatigue and improving quality of life in patients with chronic anemia who have human immunodeficiency virus (HIV) infection/AIDS or end-stage renal disease, and in those who are undergoing cancer chemotherapy.15,16 However, erythropoietin therapy is expensive, and beneficial effects may not become apparent for four to six weeks. The expense and “time to effect” for this treatment should be compared with the cost and inconvenience of transfusions in patients who have symptomatic anemia at the end of life.
Anorexia and Cachexia
Little is known about the pathogenesis of wasting syndromes. In cancer, humoral factors elaborated by the tumor appear to be involved, because cachexia can be transferred from cancer-bearing to noncancer-bearing animals in parabiotic experiments.20 It is likely that elevated levels of cytokines, notably interferons and tumor necrosis factors, play a role in the metabolic alterations observed in patients with wasting syndromes.20,21 There is no relationship between tumor size and the degree of cachexia.17
Despite the appearance of malnutrition, the wasting syndrome associated with cancer and hypermetabolic states is caused by the underlying disease process and is usually not reversible with improved nutrition. Orally or parenterally administered nutrition frequently results in increased body fat, not increased protein. The findings of a number of studies indicate that aggressive alimentation in patients with a wasting syndrome related to cancer may actually increase their discomfort.22,23 This differs for patients with AIDS.
The physician can be of particular assistance in helping the patient and family understand that cachexia is an expected consequence of the underlying disease process. Frequently, family caregivers believe that wasting is the result of their provision of inadequate care and nutrition. They can be taught to replace their “need to feed” with behaviors that alleviate symptoms, such as moistening the patient's lips and oral cavity with a sponge, offering small amounts of food as desired by the patient, providing light massage, reading to the patient or playing soft music for the patient.
Treatable causes of anorexia and cachexia in patients who are near the end of life include chronic pain, mouth conditions (dryness, mucositis resulting from chemotherapy, and infections such as oral candidiasis or oral herpes), gastrointestinal motility problems (e.g., constipation) and reflux esophagitis. In patients with cancer who are being treated with chemotherapy, radiation therapy and/or medications such as opioids or nonsteroidal anti-inflammatory drugs, an attempt should be made to determine whether anorexia and weight loss are due to mucositis, changes in gastrointestinal motility and nausea as the effects of treatment, rather than progressive disease.
In the absence of specific treatable causes, symptomatic management of cachexia at the end of life includes both nonpharmacologic and pharmacologic interventions (Table 2).14 It is important to emphasize that cachexia is part of the “normal” end-of-life process. Wasting in dying patients may result in the natural release of endorphins, causing euphoria.
Nausea and Vomiting
Nausea and vomiting can be extremely debilitating symptoms at the end of life. With available methods, effective control of these symptoms can be achieved in most patients.
The brain (chemoreceptor trigger zone, cerebral cortex, vestibular apparatus and vomiting center) and the gastrointestinal tract are the key organs involved in nausea and vomiting. Neurotransmitter receptors that mediate nausea and vomiting include those for serotonin, dopamine, acetylcholine and histamine.14 Identification of the pathophysiologic origin of nausea is helpful in prescribing effective pharmacologic interventions.
| Nonpharmacologic therapy |
| Provide patient and family education about the pathophysiology of the anorexia and cachexia in terminal illness, and the ineffectiveness of forced feeding and hydration. |
| Eliminate dietary restrictions: allow the patient to choose favorite foods and fluids, and to have them when desired and in the amount desired. |
| Reduce portion size and eliminate foods whose odor the patient finds unpleasant. |
| Explore the emotional and spiritual issues related to the patient's weight loss. |
| Pharmacologic therapy* |
| Dexamethasone (Decadron), 2 to 20 mg taken orally each morning; effect may diminish after 4 to 6 weeks of use. |
| Megestrol (Megace), 200 mg taken orally every 6 to 8 hours; titrate dosage to achieve and maintain desired effect. |
| Dronabinol (Marinol), 2.5 mg taken orally two or three times daily; titrate dosage to patient tolerance and to achieve and maintain desired effect. |
| Androgens (e.g., oxandrolone [Oxandrin], nandrolone [Durabolin]) are currently under investigation for their effects on appetite and weight. |
| Etiology | Pathophysiology | Treatment | |
|---|---|---|---|
| Metastases | |||
| Cerebral | Increased intracranial pressure, direct chemoreceptor trigger zone effect | Corticosteroids, mannitol, dopamine antagonists, histamine antagonists | |
| Liver | Toxin buildup | Dopamine antagonists, histamine antagonists | |
| Meningeal irritation | Increased intracranial pressure | Corticosteroids | |
| Movement | Vestibular stimulation (may be worse with morphine) | Acetylcholine antagonists | |
| Mentation (e.g., anxiety) | Cerebral cortex | Anxiolytics (e.g., benzodiazepines, dronabinol [Marinol]) | |
| Medications | |||
| Opioids | Chemoreceptor trigger zone, vestibular effect, gastrointestinal tract | Dopamine antagonists, histamine antagonists, acetylcholine antagonists, prokinetic agent, stimulant laxatives | |
| Chemotherapy | Chemoreceptor trigger zone, gastrointestinal tract | Serotonin antagonists, dopamine antagonists, corticosteroids | |
| Others (e.g., NSAIDs)* | Chemoreceptor trigger zone | Dopamine antagonists, histamine antagonists | |
| Mucosal irritation | |||
| NSAIDs | Gastrointestinal tract, gastritis | Cytoprotective agents | |
| Hyperacidity, gastroesophageal reflux | Gastrointestinal tract, gastritis, esophagitis, duodenitis | Antacids | |
| Mechanical obstruction | |||
| Intraluminal | Constipation, obstipation | Manage with laxatives, stool softeners, lubricants, etc. | |
| Extraluminal | Tumor, fibrotic stricture | Reversible: surgery | |
| Irreversible: manage fluids, administer corticosteroids, inhibit secretions with octreotide (Sandostatin) or scopolamine (Transderm Scop) | |||
| Motility | |||
| Ileus, opioids and other medications | Gastrointestinal tract, central nervous system | Prokinetic agent, stimulant laxatives | |
| Metabolic imbalance | |||
| Hypercalcemia, hyponatremia, hepatic or renal failure | Chemoreceptor trigger zone | Dopamine antagonists, histamine antagonists, corticosteroids; correct electrolyte imbalance | |
| Microbes | |||
| Local irritation (e.g., esophagitis, gastritis caused by infection with Candida species, Helicobacter pylori, herpesvirus, cytomegalovirus) | Gastrointestinal tract | Antibacterials, antivirals, antifungals, antacids | |
| Systemic sepsis | Chemoreceptor trigger zone | Dopamine antagonists, histamine antagonists, antibacterials, antivirals, antifungals | |
| Myocardial dysfunction | |||
| Ischemia, congestive heart failure | Vagal stimulation, cerebral cortex, chemoreceptor trigger zone | Oxygen, opioids, dopamine antagonists, histamine antagonists, anxiolytics | |
| Medication | Dosage and usual routes of administration | ||
|---|---|---|---|
| Dopamine antagonists | |||
| Haloperidol (Haldol) | 0.5 to 2 mg orally, IV or SC every 6 hours; then titrate the dosage | ||
| Prochlorperazine (Compazine) | 10 to 20 mg orally every 6 hours, 25 mg rectally every 12 hours, or 5 to10 mg IV every 6 hours | ||
| Droperidol (Inapsine) | 2.5 to 5 mg IV every 6 hours | ||
| Thiethylperazine (Torecan) | 10 to 20 mg orally every 6 hours | ||
| Perphenazine (Trilafon) | 2 to 8 mg orally or IV every 6 hours | ||
| Histamine H1 receptor blockers (antihistamines) | |||
| Diphenhydramine (Benadryl) | 25 to 50 mg orally every 6 hours | ||
| Meclizine (Antivert) | 25 to 50 mg orally every 6 hours | ||
| Hydroxyzine hydrochloride (Atarax), hydroxyzine pamoate (Vistaril) | 25 to 50 mg orally every 6 hours | ||
| Promethazine (Phenergan) | 12.5 to 25 mg IV or IM every 4 to 6 hours, or 25 mg orally or rectally every 4 to 6 hours | ||
| Acetylcholine antagonist (anticholinergic) | |||
| Scopolamine (Transderm Scop) | 1 to 3 transdermal patches every 72 hours | ||
| Serotonin antagonists | |||
| Ondansetron (Zofran) | 8 mg orally three times daily | ||
| Granisetron (Kytril) | 1 mg orally once or twice daily | ||
| Dolasetron (Anzemet) | 100 mg orally once daily | ||
| Prokinetic agent | |||
| Metoclopramide (Reglan) | 10 to 20 mg orally every 6 hours | ||
| Antacids | |||
| Liquid antacids (various) | 1 to 2 tablespoons every 2 hours as needed | ||
| Histamine H2 receptor blockers | 800 mg orally at bedtime for ulcers; 800 mg orally twice daily for gastroesophageal reflux | ||
| Cimetidine (Tagamet)* | |||
| Ranitidine (Zantac) | 150 mg orally twice daily or 300 mg orally at bedtime; decrease dosage by 50% if creatinine clearance is less than 50 mL per minute (0.84 mL per second) | ||
| Famotidine (Pepcid) | 20 to 40 mg orally at bedtime for ulcers; 20 to 40 mg orally twice daily for gastroesophageal reflux | ||
| Proton pump inhibitors | |||
| Omeprazole (Prilosec) | 20 mg orally once daily; dosage should be reduced in Asian patients | ||
| Lansoprazole (Prevacid) | 15 mg orally once daily | ||
| Cytoprotective agents | |||
| Misoprostol (Cytotec) | 200 μg orally two to four times daily | ||
| Proton pump inhibitors | Same as above | ||
| Other medications | |||
| Dexamethasone (Decadron) | 6 to 20 mg orally once daily | ||
| Dronabinol (Marinol)l | 2.5 to 5 mg orally three times daily | ||
| Lorazepam (Ativan, Intensol) | 0.5 to 2 mg orally, sublingually or SC every 4 to 6 hours | ||
| Octreotide (Sandostatin) | 50 to 150 μ g IV or SC every 8 to12 hours; titrate dosage every 24 to 48 hours to achieve and maintain desired effect | ||
If patients have persistent nausea and treatable causes have been ruled out, haloperidol (Haldol), 0.5 to 2 mg given orally, intravenously or subcutaneously every six hours, can be very effective. The dosage can be titrated, if necessary, to a total of 10 to 15 mg daily. If needed, an antihistamine or a prokinetic agent may provide additional benefit.24 Severe symptoms may require a judicious combination of agents, based on mechanism of action.
Note that health care providers should use clinical judgment and consult official prescribing information before any pharmaceutical product mentioned in this article is used.