
A more recent article on H. pylori infection is available.
Am Fam Physician. 2002;65(7):1327-1337
Helicobacter pylori is the cause of most peptic ulcer disease and a primary risk factor for gastric cancer. Eradication of the organism results in ulcer healing and reduces the risk of ulcer recurrence and complications. Testing and treatment have no clear value in patients with documented nonulcer dyspepsia; however, a test-and-treat strategy is recommended but for patients with undifferentiated dyspepsia who have not undergone endoscopy. In the office setting, initial serology testing is practical and affordable, with endoscopy reserved for use in patients with alarm symptoms for ulcer complications or cancer, or those who do not respond to treatment. Treatment involves 10- to 14-day multidrug regimens including antibiotics and acid suppressants, combined with education about avoidance of other ulcer-causing factors and the need for close follow-up. Follow-up testing (i.e., urea breath or stool antigen test) is recommended for patients who do not respond to therapy or those with a history of ulcer complications or cancer.
The spiral-shaped, gram-negative bacterium Heliocobacater pylori is found in colonized gastric mucosa or adherent to the epithelial lining of the stomach. Acute infection, acquired most likely by ingestion of the organism, is most commonly asymptomatic but may be associated with epigastric burning, abdominal distention or bloating, belching, nausea, flatulence, and halitosis.1 Virtually all patients infected with H. pylori who have had endoscopic biopsies are found to have histologic gastritis.2 H. pylori infection can lead to ulceration of the gastric mucosa and duodenum and has been found to be strongly associated with the development of epithelial and lymphoid malignancies of the stomach. It has been classified by the International Agency for Research on Cancer3 as a group I carcinogen and a definite cause of gastric cancer in humans.
H. pylori gastritis produces no symptoms in 90 percent of infected persons. The prevalence of H. pylori infection varies geographically and has been demonstrated to be as high as 52 percent in the United States.4 Factors associated with higher infection rates are increasing age, African-American or Hispanic race, lower levels of education, and birth in a developing country.
The pathogenic role of H. pylori in peptic ulcer disease, both duodenal and gastric, is well recognized—up to 95 percent of patients with duodenal ulcers and 80 percent of patients with gastric ulcers are infected.5 Eradication of the organism leads to ulcer healing and a markedly lower incidence of recurrence. The role of H. pylori in nonulcer dyspepsia has not been proved and, in most patients, eradication is not associated with improvement of symptoms.6 Most patients, however, present to the family physician with undifferentiated dyspepsia, not knowing whether an ulcer exists.
When to Test and Treat
The decision to test for the presence of H. pylori is closely tied to the intention to treat, as there is no benefit in identifying the presence of the organism without having a plan to eradicate it.7 In the absence of alarm symptoms for cancer or complicated ulcer disease (Table 1),8 the approach to testing in patients with dyspepsia (Table 2)7,9–16 can be divided into four clinical scenarios: (1) known peptic ulcer disease, currently or previously documented; (2) known nonulcer dyspepsia; (3) undifferentiated dyspepsia, and (4) gastroesophageal reflux disease (GERD).
| Older than 45 years | Jaundice |
| Rectal bleeding or melena | Family history of gastric cancer |
| Weight loss of >10 percent of body weight | Previous history of peptic ulcer |
| Anemia | Anorexia/early satiety |
| Dysphagia | |
| Abdominal mass |
| Clinical scenario | Recommended test | Levels of evidence†and comments |
|---|---|---|
| Dyspepsia* in patient with alarm symptoms for cancer or complicated ulcer (e.g., bleeding, perforation) | Promptly refer to a gastroenterologist for endoscopy. | A |
| Known PUD, uncomplicated | Serology antibody test; treat if result is positive. | A—Best evidence for eradication in presence of documented gastric or duodenal ulcer |
| Dyspepsia in patient with previous history of PUD not previously treated with eradication therapy | Serology antibody test; treat if result is positive. | A |
| Dyspepsia in patient with PUD previously treated for H. pylori | Stool antigen or urea breath test; if positive, treat with regimen different from the one previously used; retest to confirm eradication. Consider endoscopy. | B—Urea breath test should be delayed for four weeks following treatment, as acid suppression can lead to false-positive results. |
| Undifferentiated dyspepsia (without endoscopy) | Serology antibody test; treat if result is positive. | B—Supported by cost-benefit analyses and recent small RCTs |
| Documented nonulcer dyspepsia (after endoscopy) | Unnecessary | B—RCTs and meta-analyses on this topic are mixed but indicate that few patients benefit from treatment. |
| GERD | Unnecessary | B—GERD is not associated with H. pylori infection. |
| Asymptomatic with history of documented PUD not previously treated with eradication therapy | Serology antibody test; treat if result is positive. | C |
| Asymptomatic | Screening unnecessary | C |
PEPTIC ULCER DISEASE
The best evidence for the effectiveness of H. pylori eradication exists for the treatment of H. pylori-associated ulcers. In this case, treatment of H. pylori infection in patients with ulcers almost always cures the disease and reduces the risk for serious complications (e.g., perforation or bleeding).9,10 [Evidence level A, meta-analyses/RCTs]. Compared with acid suppression therapy alone, eradication of H. pylori results in a dramatic reduction in recurrence of duodenal ulcers and gastric ulcers that are not related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs)—12 percent versus 95 percent at one year.11 A previously documented peptic ulcer in a patient who has never been treated for H. pylori infection is an indication for testing and treatment.7
NONULCER DYSPEPSIA
To date, there is no convincing evidence that empiric eradication of H. pylori in patients with nonulcer dyspepsia improves symptoms. One recent meta-analysis12 showed no improvement of symptoms with H. pylori eradication, and another13 revealed a statistically significant benefit, but the effect was small, with one patient cured for every 19 treated (NNT= 19).
UNDIFFERENTIATED DYSPEPSIA
In primary care, the typical patient who presents with dyspepsia will not have had endoscopy performed and, therefore, the presence of an underlying lesion will be unknown. Symptom complexes have not been shown to predict endoscopic findings, and the high prevalence of dyspeptic symptoms makes definitive testing of all patients impractical.
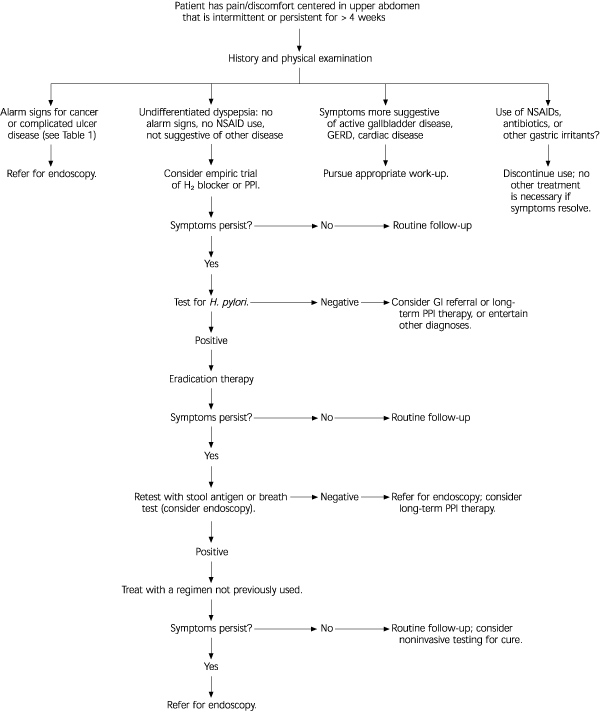
Several consensus panels have advocated a test-and-treat strategy in which patients with dyspepsia are tested for the presence of H. pylori with serology and treated with eradication therapy if the results are positive.14,15 This strategy reserves endoscopy for use in patients with alarm signs or those with persistent symptoms despite appropriate empiric therapy, and is supported by cost-benefit analysis. One such approach is shown in Figure 1. This approach is supported by cost-benefit analysis.16
GASTROESOPHAGEAL REFLUX DISEASE
H. pylori infection does not increase the risk of GERD and is actually associated with a lower severity of symptoms and a lower incidence of Barrett's esophagus. In fact, GERD-like symptoms (e.g., arising sensation of burning and regurgitation) are associated with a decreased likelihood of H. pylori infection.17 Eradication therapy does not eliminate GERD symptoms.
Helicobacter pylori Tests
| Test | What does it measure? | Sensitivity (%) | Specificity (%) | Test of cure? | Comments |
|---|---|---|---|---|---|
| Invasive (requiring endoscopic biopsy) | |||||
| Steiner's stain of gastric biopsy specimen | Histologic identification of organisms | 82 to 95 | 99 to 100 | Yes | Considered the “gold standard” |
| Rapid urease test (CLO test, Delta Wenst, Bently, Western Australia) | Urease activity of biopsy Specimen | 85 to 90 | 98 to 100 | Yes | Sensitivity reduced by acid suppression and active bleeding |
| Culture | Presence of organisms; antimicrobial sensitivities | 70 to 80 | 100 | Yes | Especially useful in research and to guide management in treatment failures; requires experienced laboratory |
| Noninvasive | |||||
| Serology: laboratory-based ELISA | IgG | 90 to 93 | 95 to 96 | No | Accurate; convenient for initial infection; titers diminish slowly after eradication and may remain positive after one year |
| Whole blood: office-based ELISA | IgG | 50 to 85 | 75 to 100 | No | Less accurate but fast, convenient, inexpensive |
| Stool: HpSA | H. pylori antigens | 95 to 98 | 92 to 95 | Yes | Relatively convenient and available |
| Urea breath test | Urease activity | 95 to 100 | 95 | Yes | Sensitivity reduced by acid suppression |
| String test (swallowed and recovered polymeric string) | Culture or polymerase chain reaction on gastric mucus | 75 to 80 | 75 to 100 | No | Minimally invasive method to obtain viable organisms, but retrieval rate less than with endoscopy |
| Urine ELISA | IgG | 70 to 96 | 77 to 85 | No | Greater patient acceptance and convenience than stool test; not yet readily available |
| Saliva ELISA | IgG | 82 to 91 | 71 to 85 | No | Greater patient acceptance and convenience than stool test; not yet readily available |
ENDOSCOPY AND BIOPSY
Alarm symptoms for cancer or ulcer complication warrant prompt endoscopic evaluation. A Steiner's stain for microscopic examination of gastric antral biopsy specimens is considered the gold standard for detecting the presence of H. pylori. A rapid urease test is highly specific and simple, and can also be performed on biopsy samples; however, it may have false-negative results, particularly if the patient has recently taken a proton pump inhibitor (PPI).20 Cultures of biopsy specimens obtained during endoscopy can be tested for antimicrobial resistance in cases of treatment failure. If necessary, a specimen for culture can also be obtained using a string test, a less invasive but also less reliable technique in which a highly absorbent polymer string is partially swallowed and then removed manually to recover gastric material.21
SEROLOGY/ELISA
When endoscopy is not performed, the most commonly used diagnostic approach is the laboratory-based serologic antibody test. This enzyme-linked immunosorbent assay (ELISA) detects IgG antibodies to H. pylori, indicating current or past infection. Because H. pylori infection is not known to spontaneously resolve, a positive serologic test suggests active infection in patients who have not undergone eradication therapy. The serologic test results may or may not revert to negative once the organism is eradicated; therefore, the test is not used to identify persistent infection, although a negative test result does reliably identify cure.22
Although they are convenient and inexpensive, rapid, office-based, whole-blood tests are less accurate than laboratory-based serologic tests.20
UREA BREATH TEST
The urea breath test is a reliable test for cure and can detect the presence or absence of active H. pylori infection with greater accuracy than the serologic test. It is usually administered in the hospital outpatient setting because it requires time and special equipment. Breath tests involve patient consumption of carbon 13- or carbon 14-labeled urea. The bacterium metabolizes urea rapidly, and the labeled carbon is absorbed into the patient's circulation. In 15 to 20 minutes, the labeled carbon dioxide in an exhaled breath sample can be measured. As with other tests of urease activity, false-negative results can result from acid suppression with PPIs23; therefore, acid suppression therapy should be withheld for two weeks before the test is administered and for at least four weeks following completion of eradication therapy if the test is to be used to check for cure.
NEWER TESTS
Several new, noninvasive tests of stool, saliva, and urine samples are being investigated. The most promising of these is an enzyme-linked immunoassay detecting H. pylori antigen in stool specimens. Highly sensitive and specific, the stool antigen test reverts to negative from five days to a few months after eradication of the organism, with 90 percent specificity.24,25 This test, which is currently available, is useful in confirming eradication, and, because it is office-based, is less costly and more convenient than the urea breath test. False-positive results may occur even four weeks following eradication therapy.
Principles of Treatment
Antimicrobial resistance and incomplete treatment are major reasons for treatment failure.26,27 The treatment of H. pylori infection can be likened to the treatment of tuberculosis because multidrug regimens and an adequate length of treatment are needed to eradicate the organism. Because of the lengthy period of therapy, convenience and tolerability become important considerations in choosing a treatment plan.28 While success with shorter durations of treatment has been reported, continued therapy for 14 days is the most reliable and effective regimen and is recommended in the United States.7
PHARMACOLOGIC THERAPY
Table 4 provides a practical list of selected effective drug combinations used for treating patients with H. pylori infection. Only triple and quadruple therapies with reported eradication rates approaching 90 percent or more are included. Single and dual drug therapies have unacceptably low cure rates and are not recommended. Because microbial resistances are rapidly developing, regional surveillance may become important in determining the best treatment courses in the future.
| Treatment (10 to 14 days of therapy recommended) | Cost | Convenience factor | Tolerability | ||
|---|---|---|---|---|---|
| Triple therapy | |||||
| 1. Omeprazole (Prilosec), 20 mg two times daily | $260 (LAC†) | Twice-daily dosing | Fewer significant side effects, but more abnormal taste versus other regimens | ||
| or | |||||
| Lansoprazole (Prevacid), 30 mg two times daily | 195 (LAC†‡) | ||||
| plus | |||||
| Metronidazole (Flagyl), 500 mg two times daily | 200 (OAC) | ||||
| or | |||||
| Amoxicillin, 1 g two times daily | 194 (LMC) | ||||
| plus | |||||
| Clarithromycin (Biaxin), 500 mg two times daily | 199 (OMC) | ||||
| 2. Ranitidine bismuth citrate (Tritec), 400 mg twice daily | 118 (RCT) | Twice-daily dosing | Increased diarrhea versus other regimens | ||
| plus | |||||
| Clarithromycin, 500 mg twice daily | |||||
| or | |||||
| Metronidazole, 500 mg twice daily | 136 (RCA) | ||||
| plus | |||||
| Tetracycline, 500 mg twice daily | 73 (RMT) | ||||
| or | |||||
| Amoxicillin, 1 g twice daily | 92 (RMA) | ||||
| Quadruple therapy | |||||
| 3. Bismuth subsalicylate (Pepto Bismol), 525 mg four times daily/2 tablets four times daily | 142 (BMT§ plus H2R†) | 18 pills daily | More side effects; increased nausea versus other regimens | ||
| plus | |||||
| Metronidazole, 250 mg four times daily | 87 (BMT [separately] plus H2R†) | ||||
| plus | |||||
| Tetracycline, 500 mg four times daily | |||||
| plus | |||||
| H2RA for 28 days | |||||
| 4. Bismuth subsalicylate, 525 mg four times daily/2 tablets four times daily | 206 (BMT plus PPI) | 18 pills daily | Increased nausea | ||
| plus | |||||
| Metronidazole, 250 mg four times daily | |||||
| plus | |||||
| Tetracycline, 500 mg four times daily | 153 (BMT separately] plus PPI) | ||||
| plus | |||||
| PPI for 14 days | |||||
Antibiotics
Amoxicillin, a semi-synthetic penicillin, is an effective antibiotic for H. pylori infection. The frequency of amoxicillin-resistant H. pylori organisms is low. The drug rapidly accumulates in antral mucosa via systemic circulation. Its antimicrobial activity against H. pylori depends on the pH level; the minimal inhibitory concentration (MIC) decreases as the pH increases.29 Clarithromycin is also quite effective, although more resistant organisms are emerging.30 Co-administration with a PPI significantly increases the concentration of clarithromycin in the antral mucosa and the mucus layer. Erythromycin and azithromycin are much less effective macrolides in vivo and should not be used in H. pylori treatment.31 Metronidazole is active against H. pylori, and its bioavailability is not influenced by acid suppression; however, resistance to metronidazole is high.31 Furazolidone has been described as an alternative to metronidazole in resistant cases but, as a monoamine oxidase inhibitor, it may be associated with food and drug interactions.31
Acid Reducers
The H. pylori organism prefers an acidic environment. Increasing the gastric pH with the use of a histamine H2-receptor antagonist (H2RA) or a PPI has been shown to improve the effectiveness of antibiotic therapy.32 In the presence of ulcer disease, PPIs have largely replaced H2RAs because of their ability to provide more rapid pain relief and better control of pH. In addition, PPIs have demonstrated antimicrobial activity against H. pylori.33 It appears that all the PPIs are comparable; however, larger head-to-head comparisons are not available.
Bismuth Compounds
Bismuth salts have no substantial acid-neutralizing capacity but inhibit pepsin, increase secretion of mucus, and form a barrier to the diffusion of acid in the ulcer crater. They also cause detachment of H. pylori from the gastric epithelium and disrupt bacterial cell walls, resulting in lysis of the bacterium. Side effects include darkening of the oral cavity and stool. Ranitidine bismuth citrate is a combination salt with intrinsic antisecretory and antimicrobial activity that is effective in combination with antibiotics in the eradication of H. pylori. It is not effective as monotherapy.34
Because patient adherence to therapy is critical, simpler regimens with twice-daily dosing may be more successful in eradicating the H. pylori organism. Based on efficacy, PPI triple therapy or bismuth quadruple therapy for 14 days are recommended in the United States as first-line treatments for patients with H. pylori infection. PPI quadruple therapy or a regimen including furazolidone may serve as second-line treatment for eradication of initial failures and in cases of metronidazole resistance.31,35
Patient Education
In addition to H. pylori eradication therapy, patients should be counseled to avoid other factors that increase their risk of dyspepsia and peptic ulcer disease. The use of NSAIDs and tobacco increase the risk of peptic ulcer disease, particularly of the stomach.36 While it is hypothesized that both H. pylori and NSAIDs disrupt the integrity of the stomach lining, results of studies have not demonstrated an interaction between H. pylori and NSAID use in the development of ulcerations. NSAIDs are known to delay ulcer healing, however, and NSAID therapy should be stopped regardless of the underlying cause of the ulcer.37 Smoking appears to have a synergistic relationship with H. pylori and should also be stopped.
Patient education about the need for effective eradication therapy and the necessity of completing the initial drug regimen is critical. A follow-up plan must be emphasized because further diagnostic testing may be needed to ensure eradication of the H. pylori organism, particularly if symptoms persist.
Post-treatment Follow-Up
Patients with a history of ulcer complications, gastric mucosa-associated lymphoid tissue (MALT), or early gastric cancer should undergo a routine post-treatment urea breath test or endoscopy to ensure successful eradication. These patients will usually be followed in collaboration with a gastroenterologist. Routine, noninvasive follow-up testing also can be considered in patients who have persistent symptoms following eradication therapy. In these patients, the stool antigen test, performed four weeks following therapy, is a convenient alternative. Because of the risk of ulcer recurrence and the potential for malignant transformation caused by H. pylori infection, follow-up is important—whether through testing or watchful waiting under the presumption that a long symptom-free period indicates cure.
Serology is not practical as a test for cure because it can take more than one year to revert to negative; however, a negative result is predictive of successful eradication. To date, good evidence does not exist to support routine laboratory testing for cure in patients whose symptoms respond to eradication therapy for uncomplicated ulcer disease or undifferentiated dyspepsia.
TREATMENT FAILURE
Patients with persistent H. pylori infection despite initial therapy should be retreated using an alternate combination regimen. Data from one study38 in which patients were treated with quadruple therapy that included lansoprazole (30 mg twice daily), tetracycline (500 mg four times daily), metronidazole (500 mg three times daily) and bismuth sub-citrate (120 mg four times daily) for one week resulted in eradication in 20 of 21 patients who had not responded to triple therapy. When treatment fails a second time, patients should be referred to a gastroenterologist.