
Am Fam Physician. 2002;65(8):1615-1621
Antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis is the most common primary systemic small-vessel vasculitis to occur in adults. Although the etiology is not always known, the incidence of vasculitis is increasing, and the diagnosis and management of patients may be challenging because of its relative infrequency, changing nomenclature, and variability of clinical expression. Advances in clinical management have been achieved during the past few years, and many ongoing studies are pending. Vasculitis may affect the large, medium, or small blood vessels. Small-vessel vasculitis may be further classified as ANCA-associated or non-ANCA–associated vasculitis. ANCA–associated small-vessel vasculitis includes microscopic polyangiitis, Wegener's granulomatosis, Churg-Strauss syndrome, and drug-induced vasculitis. Better definition criteria and advancement in the technologies make these diagnoses increasingly common. Features that may aid in defining the specific type of vasculitic disorder include the type of organ involvement, presence and type of ANCA (myeloperoxidase–ANCA or proteinase 3–ANCA), presence of serum cryoglobulins, and the presence of evidence for granulomatous inflammation. Family physicians should be familiar with this group of vasculitic disorders to reach a prompt diagnosis and initiate treatment to prevent end-organ damage. Treatment usually includes corticosteroid and immunosuppressive therapy.
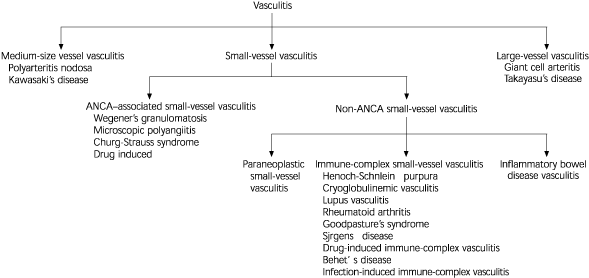
Vasculitis is a process caused by inflammation of blood vessel walls and results in a variety of disorders. A good and accepted classification system for vasculitis has not emerged, although it may be categorized by the size or type of the involved blood vessel as large-, medium-, or small-vessel vasculitis.1 Small-vessel vasculitis is defined as vasculitis that affects vessels smaller than arteries (i.e., arterioles, venules, and capillaries); however, small-vessel vasculitis can also involve medium-sized arteries.1
Figure 11–3 depicts some major classifications of vasculitis and illustrates the position of antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis among other types. It is the most common cause of small-vessel vasculitis and includes microscopic polyangiitis, Wegener's granulomatosis, Churg-Strauss syndrome, and certain types of drug-induced vasculitis.1,3
What are ANCA?
ANCA are specific antibodies for antigens in cytoplasmic granules of neutrophils and monocyte lysosomes, first reported in 1982.4 These antibodies can be detected with indirect immunofluorescence microscopy. Two major patterns of staining are present: cytoplasmic ANCA (c–ANCA) and peri-nuclear ANCA (p–ANCA). Specific immunochemical assays demonstrate that c–ANCA is mainly antibodies to proteinase 3, and p–ANCA is antibodies to myeloperoxidase. Using antigen-specific immunochemical assay to characterize ANCA (rather than the pattern of immunofluorescence microscopy) is more specific and more clinically relevant; therefore, the terms proteinase 3-ANCA (PR3-ANCA) and myeloperoxidase–ANCA (MPO–ANCA) are now in use.4
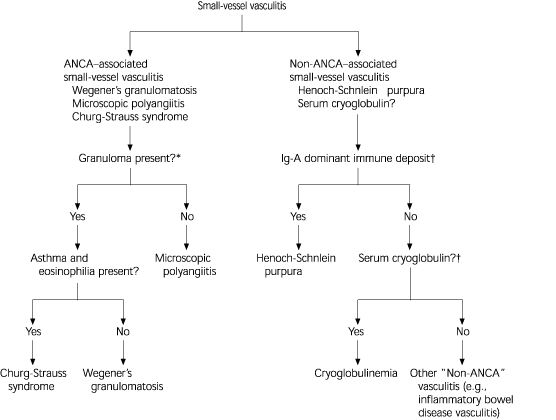
Figure 23 illustrates the differential diagnosis of ANCA and non–ANCA-associated vasculitis. About 10 percent of patients with microscopic polyangiitis (the most common type of ANCA–associated small vessel vasculitis) and Wegener's granulomatosis have negative assays for ANCA; however, this finding does not completely rule out these diseases and ANCA titers do not always correlate with disease activity.3 On the other hand, a positive ANCA assay result is not solely diagnostic of ANCA–associated vasculitis.
Diagnosis of Small-Vessel Vasculitis
Small-vessel vasculitis has common features that should alert the family physician to consider its presence. Three questions should be asked in this regard and are addressed in this article: (1) “Is small-vessel vasculitis present?”; (2) “Which specific type of small-vessel vasculitis is present?” and (3) “Is the diagnosis of the specific type of small-vessel vasculitis important?”
IS SMALL-VESSEL VASCULITIS PRESENT?
Table 15–8 summarizes the potential clinical manifestations of ANCA-associated small-vessel vasculitis that is generally shared by most types of small-vessel vasculitis. Small-vessel vasculitis should be suspected in any patient who presents with a multisystem disease that is not caused by an infectious or malignant process (e.g., renal dysfunction, skin rashes, pulmonary manifestations, or neurologic manifestation).5–8 Constitutional symptoms are common. The frequency and combination of various system involvements vary among individual disease entities.
| System | Manifestations |
|---|---|
| Constitutional | Fever, weight loss, anorexia, general malaise |
| Musculoskeletal | Myalgia, arthralgia |
| Skin | Palpable purpura, urticaria |
| Kidneys | Proteinuria, hematuria, renal insufficiency, renal failure, necrotizing glomerulonephritis |
| Respiratory tract | Dyspnea, cough, hemoptysis; lung infiltrate, interstitial lung disease, pulmonary hemorrhage |
| Nervous system | Peripheral neuropathy, especially mononeuritis |
| Gastrointestinal tract | Fecal blood, elevated liver enzymes; diarrhea, nausea, vomiting, abdominal pain |
The most common cutaneous lesion is palpable purpura—a slightly raised, non blanching eruption that usually begins in the lower extremities.3,5 Occasionally, the rash is vesicular or slightly ulcerated. Urticaria can also be a manifestation of small-vessel vas-culitis. Unlike nonvasculitic allergic urticaria, vasculitic urticaria lasts more than one day and may evolve into purpuric lesions. The presence of hypocomplementemia may indicate that the vasculitis is immune complex-mediated rather than ANCA-associated primary vasculitis.3
Renal involvement in vasculitis may progress to renal failure. Results of biopsy of the kidney commonly reveals glomerulonephritis. Focal necrosis, crescentic formation and the absence or paucity of immunoglobulin deposits characterize glomerulonephritis in patients with ANCA-associated vasculitis.6
It is important, however, to differentiate small-vessel vasculitis from other diseases that result in multisystem manifestations. Diseases with widespread embolization to different organs (e.g., atheroembolic disease, endocarditis, antiphospholipid syndrome, and atrial myxoma) can produce similar clinical presentations.9 Persons with sepsis can also present with multisystem involvement. It is also important to realize that small-vessel vasculitis may be secondary to infections or malignancy. Some viral, bacterial, and fungal infections may be complicated by vasculitis, which is predominantly a dermal vasculitis.9 The diagnosis is suggested by the clinical history. Malignancy, such as lymphomas, leukemia, myeloprolifera-tive, and myelodysplastic syndromes may be associated with vasculitis; however, solid tumors are less commonly associated with vasculitis.9 It must be emphasized that underlying infectious or malignant causes should be thoroughly evaluated before the diagnosis of primary vasculitis is made—even if the ANCA assay result is positive.
WHAT SPECIFIC TYPE OF SMALL-VESSEL VASCULITIS IS IT?
Table 23,9 depicts some of the clinical features that may help in the diagnosis of the specific type of vasculitis. Laboratory assessment should include a complete blood cell count and routine chemistry profile, urinalysis, fecal occult blood test, and chest radiography.9 There may be normocytic anemia, thrombo-cytosis, elevated erythrocyte sedimentation rate, increased liver function, or evidence of renal involvement. ANCA serum levels should also be measured. Other laboratory tests that should be performed to exclude ANCA-associated small-vessel vasculitis include antinu-clear antibody, rheumatoid factor, cryoglobulins, complement, antibodies to hepatitis B and C, and human immunodeficiency virus (HIV) testing.9 Chest and sinus computed tomographic scans may also be performed, if appropriate. Angiography may reveal evidence of medium- or large-vessel vasculitis. Pathologic examination of the involved tissue (e.g., skin, nerve, lung, or kidney) may aid in documenting the type of small-vessel vasculitis. Biopsy should be obtained from symptomatic and accessible sites. Biopsies from asymptomatic sites have a low yield of positive results.1,8,9
| Clinical features | Probable type of small-vessel vasculitis |
|---|---|
| Pulmonary and renal symptoms | Wegener's granulomatosis |
| Microscopic polyangiitis | |
| Pulmonary-dermal symptoms | Cryoglobulinemia |
| Henoch-Schönlein purpura | |
| Asthma and eosinophilia | Churg-Strauss syndrome |
| Upper respiratory tract involvement (e.g., sinusitis and otitis media) | Wegener's granulomatosis |
ANCA-associated small-vessel vasculitis is the most common primary type of vasculitis in older adults, while Henoch-Schönlein purpura is most common in children.3
Wegener's Granulomatosis
Wegener's granulomatosis commonly has the classic triad of involvement of the upper respiratory tract, lungs, and kidneys.9,10 Upper respiratory tract signs and symptoms include sinusitis, nasal ulcers, otitis media, or hearing loss. Upper respiratory tract signs and symptoms are seen in 70 percent of patients and pulmonary infiltrates or nodules that may cavitate develop in 85 percent of patients.9,10 Serum antiprotease 3–ANCA (c–ANCA) is positive in 75 to 90 percent, although 20 percent may have positive p–ANCA Open lung biopsy is the most definitive diagnostic test. Sinus biopsy is diagnostic in only 30 percent of cases because inflammatory findings are often nonspecific and renal biopsy is also relatively nonspecific.9,10 Wegener's granulomatosis can affect patients at any age, with the peak incidence during the fourth decade of life and is slightly more common in men.10
Microscopic Polyangiitis
Microscopic polyangiitis is the most common ANCA–associated small-vessel vasculitis, and is characterized by the presence of ANCA and few or no immune deposits in the involved vessels.1,3,7,9 The kidneys are the most commonly affected organs in 90 percent of patients who have this type of vasculitis.3,9 Patients present with variable combinations of renal manifestations, palpable purpura, abdominal pain, cough, and hemoptysis.7 Most patients have positive MPO–ANCA (p–ANCA), although PR3–ANCA (c–ANCA) may be also present in 40 percent of patients.9 The most common age of onset is 40 to 60 years and is more common in men.9
Churg-Strauss Syndrome
Churg-Strauss syndrome is a rare disease and has three phases: allergic rhinitis and asthma, eosinophilic infiltrative disease resembling pneumonia, and systemic small vessel vasculitis with granulomatous inflammation.8 The vasculitic phase usually develops within three years of the onset of asthma. Almost all patients have more than 10 percent eosinophils in the blood. Coronary arteritis and myocarditis are the principal causes of morbidity and mortality.8 The age of onset varies from 15 to 70 years and is more common in men.8
Drug-Induced Vasculitis
Drug-induced vasculitis usually develops within seven to 21 days after a drug is started and may be confined to the skin.3 Skin lesions are identical to those seen in systemic small vessel vasculitis. Drugs cause approximately 10 percent of vasculitic skin lesions. Drugs that have been implicated include penicillin, aminopenicillins, sulfonamides, allopurinol, thiazides, quinolones, hydantoins, and propylthiouracil.3 Some drugs, such aspropylthiouracil and hydralazine (Apresoline), appear to cause vasculitis by inducing ANCA.3
IS THE DIAGNOSIS OF THE SPECIFIC TYPE OF SMALL-VESSEL VASCULITIS IMPORTANT?
A rapid diagnosis of ANCA-associated small-vessel vasculitis is critically important, because life-threatening injury to organs often develops quickly and is mitigated dramatically by immunosuppressive therapy. On the other hand, because the treatment of patients with microscopic polyangiitis or Wegener's granulomatosis is essentially the same when there is major organ injury, it is unnecessary to distinguish conclusively between these closely related variants of ANCA-associated small-vessel vasculitis before initiating treatment.1,11
Treatment
Treatment of patients with microscopic polyangiitis and Wegener's granulomatosis has three phases: (1) induction of remission, (2) maintenance of remission, and (3) treatment of relapse.11 Current induction therapy often consists of cyclophosphamide (Cytoxan) and corticosteroids. For aggressive disease, use of high-dose intravenous methylprednisolone for three days is recommended, combined with intravenous or oral cyclophosphamide.11 Tapering doses of prednisone should follow, along with cyclophosphamide maintenance for 12 to 18 months. The lowest dosage of steroids that controls the disease should be used, and infection should be considered if the symptoms appear to exacerbate. For patients in sustained remission at 12 months, the use of all medications may be gradually discontinued. Patients whose symptoms are under good control must, nevertheless, be closely followed at six-month intervals for signs and symptoms of relapse. During treatment with these agents, complete blood counts and liver function tests should be performed periodically.11
Other treatment regimens that may be of benefit include methotrexate, azathioprine (Imuran), trimethoprim-sulfamethoxazole (Bactrim, Septra), plasma exchange, cyclosporine (Sandimmune), intravenous im-munoglobulin, and monoclonal antibodies.11
During the use of potentially ulcerogenic immunosuppressive therapy, patients may be given H2-blockers or proton-pump inhibitors. Prophylactic treatment with fluconazole (Diflucan) orally for fungal infection may be considered, as well as trimethoprim-sulfamethoxazole (480 mg) three times weekly for prophylactic treatment of patients with pneumocystis carinii prophylaxis.11
Patients with Churg-Strauss syndrome usually respond to high-dose corticosteroid therapy alone, although some cases may require the addition of cytotoxic drugs.11
Comorbid conditions that accelerate vascular damage, such as hypertension, diabetes, hypercholesterolemia, and smoking should be appropriately controlled.
In drug-induced vasculitis, the offending agent should be stopped. Antihistamines and nonsteroidal anti-inflammatory drugs help alleviate skin discomfort and reduce associated arthralgias and myalgias. Severe cutaneous disease may warrant oral corticosteroid therapy.1