
Am Fam Physician. 2002;65(9):1834-1841
Neurologic complications continue to pose problems in patients with metastatic prostate cancer. From 15 to 30 percent of metastases are the result of prostate cancer cells traveling through Batson's plexus to the lumbar spine. Metastatic disease in the lumbar area can cause spinal cord compression. Metastasis to the dura and adjacent parenchyma occurs in 1 to 2 percent of patients with metastatic prostate cancer and is more common in those with tumors that do not respond to hormone-deprivation therapy. Leptomeningeal carcinomatosis, the most frequent form of brain metastasis in prostate cancer, has a grim prognosis. Because neurologic complications of metastatic prostate cancer require prompt treatment, early recognition is important. Physicians should consider metastasis in the differential diagnosis of new-onset low back pain or headache in men more than 50 years of age. Spinal cord compression requires immediate treatment with intravenously administered corticosteroids and pain relievers, as well as prompt referral to an oncologist for further treatment.
Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men.1 Histologic evidence of prostate adenocarcinoma is present in 30 percent of men more than 50 years of age and in 70 percent of men more than 80 years old. About 9.5 percent of men will have a clinical diagnosis of prostate cancer in their lifetime, and 2.9 percent will succumb to this malignancy.2,3
Although most men with prostate cancer have asymptomatic, indolent disease, central nervous system (CNS) complications often occur with advanced metastatic disease4,5 (Table 1).4–6 CNS involvement may present as back pain caused by spinal cord compression resulting from bone metastasis via the paravertebral venous plexus or, less commonly, as headache or neurologic changes caused by the hematogenous spread of prostate cancer to the brain. Paraneoplastic syndromes, including neuropathies (sensory, peroneal, etc.), cerebellar ataxia, and limbic and brainstem encephalitides, may also occur; discussion of these rare complications is beyond the scope of this article.7,8
| Complication (incidence, %) | Clinical clues (incidence, %) | Treatment options |
|---|---|---|
| Spinal cord compression caused by metastasis (7) |
|
|
| Brain metastasis (1 to 2) |
|
|
Lesions in the brain and spinal cord require prompt treatment. Hence, family physicians need to consider metastatic prostate cancer in the differential diagnosis of new-onset back pain or headache in men more than 50 years of age.
Anatomy and Metastasis of Prostate Cancer
The pudendal nerve innervates the few striated muscles within the prostatic capsule. The parasympathetic nerves emanate from S2 to S4 and form the pelvic nerve. The sympathetic preganglionic nerves, which reside in the thoracolumbar region between T6 and L2, provide the major neural input to the prostate and reach the pelvis through the hypogastric nerve (Figure 1).
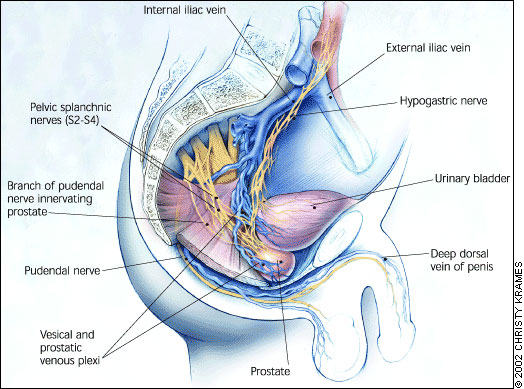
Prostate cancer has been shown to metastasize by following the venous drainage system through the lower paravertebral plexus, or Batson's plexus.4,9 Although hematogenous spread of other malignancies is most commonly to the lungs and liver, 90 percent of prostatic metastases involve the spine, with the lumbar spine affected three times more often than the cervical spine. Prostate cancer also spreads to the lungs in about 50 percent of patients with metastatic disease, and to the liver in about 25 percent of those with metastases.4
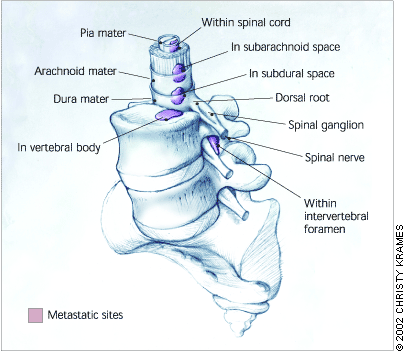
Epidural metastases are the result of contiguous spread from lesions of the calvaria to the meninges. Because of the protective layer of the dura mater, subdural and intra-parenchymal metastases from prostate cancer are rare (Figure 2).
Spinal Metastasis
SYMPTOMS
The symptoms of spinal cord compression include progressive radicular pain that is aggravated by movement. This pain can be confused with the pain caused by an osteodegenerative process of the spine. However, reclining does not alleviate back pain in patients with spinal cord compression resulting from metastatic prostate cancer. Back pain is present in nearly all patients with prostate cancer that has metastasized to the spine. Percussion over the involved vertebral body may evoke tenderness.
Muscle weakness evolves over a few days to weeks after the initial pain. Weakness usually affects the proximal muscles of the lower extremities and may or may not involve sensory loss. Autonomic dysfunction can cause urinary retention or, less frequently, bladder or bowel incontinence.
DIAGNOSIS
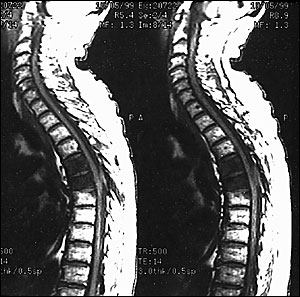
In 85 to 90 percent of patients with epidural cord compression, plain-film radiographs of the spine detect abnormalities such as collapse or erosion of the vertebral body or pedicle, but these findings are not specific.10 If spinal cord compression is suspected, magnetic resonance imaging (MRI) should be performed on an emergency basis (Figure 4). If MRI is not available, computed tomographic (CT) myelography can be used. Neuroimaging of the entire spine is necessary because epidural tumors may develop at different levels of the spine.11
Cerebrospinal fluid (CSF) examination is not very helpful and is rarely required for the diagnosis of spinal cord compression if MRI is available. CSF examination has a high false-negative rate for the detection of malignant cells.
The serum prostate-specific antigen (PSA) level is highly predictive of bone metastasis. If the serum PSA level is above 100 ng per mL, the positive predictive value is 74 percent. If the serum PSA level is less than 10 ng per mL, the negative predictive value is 98 percent.12 The CSF PSA level may prove useful for identifying intradural metastasis of prostate cancer in patients with an as yet unestablished primary tumor or with multiple malignancies. The medical literature contains a report of a 79-year-old man with lumbosacral pain who repeatedly had normal serum PSA levels and neuroimaging studies, but a CSF PSA level that was elevated to 29 ng per mL; MRI studies ultimately detected spinal metastasis from prostate cancer.13
TREATMENT
Treatment should be initiated as soon as spinal cord compression is diagnosed. With prompt treatment, there is an 89 to 100 percent likelihood of preserved ambulation in patients who present without walking difficulties. The likelihood of subsequent ambulatory function drops to 39 to 83 percent in patients who present with impaired ambulation, and to 10 to 20 percent in those who present with paralysis.14,15
Treatment involves reducing or alleviating pain as well as maintaining overall neurologic function. By using a combination of pharmaceutical and nonpharmaceutical modalities, physicians can achieve pain control in 85 to 95 percent of patients.16 Opioid medication is the mainstay of therapy for patients with severe, debilitating pain. Regimens using morphine, hydromorphone (Dilaudid), fentanyl (Duragesic), and oxycodone (Roxicodone) should follow the analgesic “ladder” developed by the World Health Organization, with rescue doses of an opioid available to manage breakthrough pain.16,17
Intravenously administered corticosteroids help to decrease cord edema and pain, retain motor function, and improve outcome after treatment. In one placebo-controlled study,18 corticosteroids improved ambulatory function from 63 percent to 81 percent in patients with high-grade radiologic lesions. After six months, 59 percent of the steroid-treated patients still ambulated, compared with 33 percent of placebo-treated patients; however, median survival remained equal. Nonetheless, dexamethasone sodium phosphate (Decadron) is the treatment of choice in patients with spinal cord compression caused by metastatic prostate cancer.
The dosing of dexamethasone is somewhat controversial. Depending on the severity of the lesion, investigators have recommended an intravenous bolus dose (loading dose) ranging from 16 to 100 mg, followed by 4 to 24 mg given intravenously four times daily for three days; tapering is accomplished by reducing the dosage by one third every three days during radiotherapy.19 High-dose dexamethasone therapy with a bolus dose of 96 to 100 mg has side effects that outweigh the benefits over use of a 16-mg loading dose with a 14-day taper.20,21 Furthermore, the 16-mg regimen does not have a significant difference in the number of patients having pain, bladder dysfunction, or inability to walk.20,21
Surgical decompression is usually reserved for patients with a solitary spinal lesion (which is seldom the situation in metastatic prostate cancer). In hormone-naïve patients, corticosteroids and androgen ablation therapy are given priority, followed by radiotherapy in patients who become refractory to corticosteroids and hormone-deprivation therapy. At this juncture, a medical oncologist should be involved.
The treatment of spinal cord compression generally improves motor strength and function in patients with metastatic prostate cancer. However, there is a 45 percent risk of another episode of compression at the same site or a new site within two years.11
Brain Metastasis
Brain metastasis is rare in prostate cancer and occurs late in the course of the disease. It usually represents the failure of hormone-deprivation therapy and the presence of disseminated disease.
Data collected before the importance of PSA values was recognized indicate that the average time from the diagnosis of prostate cancer to the occurrence of metastasis is 19 months for bone metastasis, 35 months for lung metastasis, and 60 months for brain metastasis.22,23 The long time between diagnosis and brain involvement strongly favors the cascade theory of tumor spread. Metastasis to the brain can occur by way of Batson's plexus or by direct extension from adjacent structures such as the sphenoid bone or sinuses.24
The most common intracranial sites of prostate cancer metastasis are the leptomeninges (67 percent), cerebrum (25 percent), and cerebellum (8 percent).22 Other primary cancers, such as lung and breast tumors, are more likely to have intraparenchymal metastases than leptomeningeal involvement.
SYMPTOMS
Patients rarely present with neurologic symptoms as the first manifestation of prostate cancer. Presentation with a solitary brain metastasis as the only site of prostate cancer spread is even more rare. Leptomeningeal metastasis (or carcinomatosis) is usually clinically silent, although it can present with deficits in multiple anatomic sites.25
DIAGNOSIS
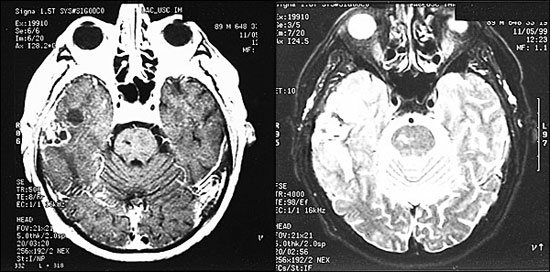
No test other than gadolinium-enhanced MRI is required to exclude or confirm the presence of brain metastases. Compared with CT scanning, MRI is more sensitive in detecting multiple metastases, especially at the gray-white junction.26
TREATMENT
Unless seizures occur, the use of prophylactic anticonvulsants, particularly phenytoin (Dilantin), is not encouraged.27,28 In combination with radiotherapy, phenytoin may cause Stevens-Johnson syndrome (erythema multiforme major).28 Dexamethasone therapy should be started early, and referral to an oncologist is warranted.
A two-week course of radiotherapy is the most common treatment for patients with multiple brain metastases or leptomeningeal involvement. Surgical removal of a solitary lesion usually extends survival.29
Various stereotactic radiosurgical techniques, including the proton beam, gamma knife, linear accelerator (LINAC) X-knife and multileaf collimators with intensity modulators, are becoming more widely available. Because these modalities provide a precise beam of radiation, damage to surrounding normal tissue is limited.30