
Am Fam Physician. 2002;65(10):2049-2057
A more recent article on management of infants born to mothers with HIV infection is available.
The management of infants whose mothers are infected with the human immunodeficiency virus (HIV) involves minimizing the risk of vertical transmission of HIV, recognizing neonatal HIV infection early, preventing opportunistic infections, and addressing psychosocial issues. Maternal antiretroviral drug therapy during pregnancy and labor, followed by six weeks of neonatal zidovudine therapy, can significantly decrease the risk of vertical transmission. Additional antiretroviral drugs may be needed in some high-risk newborns. Elective cesarean section also may prevent vertical transmission of HIV. Virologic tests allow early diagnosis of HIV infection, facilitating the timely initiation of aggressive treatment and the prevention of opportunistic infections. Even when tests are negative, infants must be closely monitored until age 18 months to completely rule out HIV infection. Prophylaxis forPneumocystis carinii pneumonia should be initiated when HIV-exposed infants are six weeks old and should be continued for at least four months, regardless of negative virologic tests, becauseP. carinii pneumonia is often the initial presentation of HIV infection in infants. Laboratory monitoring, screening for perinatal infections, appropriate social support, and other modifications of standard infant care are also necessary.
Currently, more than 160,000 women of childbearing age in the United States may be infected with the human immunodeficiency virus (HIV).1 By 1995, 16,000 U.S. infants had become infected with HIV perinatally, and the leading cause of death in young children was the acquired immunodeficiency syndrome (AIDS).2 Before 1995, perinatally infected infants had a 50 percent chance of developing AIDS by three years of age and a 90 percent chance of dying by 10 years of age.3 From 1992 to 1997, the number of AIDS cases in children declined by 66 percent, primarily because major advances in management resulted in decreased vertical transmission of HIV during pregnancy.2 The risk of perinatal HIV infection can vary from 1 to 33 percent, depending on interventions and maternal disease state.4
The early identification of HIV infection, the administration of highly active antiretroviral drug therapy, the suppression of viral loads to undetectable levels, and the prevention of opportunistic infections all have been shown to prolong life and prevent morbidity in adults. In theory, the principle of viral load suppression would apply to infants as well as adults. However, the management of HIV infection in children is a rapidly evolving area with limited data.
Unique considerations in infants born to HIV-infected mothers include in utero exposure to antiretroviral drugs, perinatal exposure to HIV and other infections, differences in diagnosis and disease manifestations compared with older patients, altered antiretroviral pharmacodynamics, and medication adherence issues such as poor palatability.
The management of HIV infection is subject to frequent changes. The basic principles of caring for infants exposed to or infected with HIV have been outlined in guidelines from several organizations and consensus panels.4–7 HIV management guidelines are regularly updated on the HIV/AIDS Treatment Information Service Web site (http://www.hivatis.org).
Natural Course of HIV Infection in Infants
| Maternal factors | Intrapartum events |
| Low CD4+ lymphocyte count High viral load Advanced AIDS Preterm delivery Chorioamnionitis Presence of p24 core antigen | Instrumental delivery Use of fetal scalp monitor Fetal scalp pH measurement Use of DeLee suctioning Artificial rupture of membranes Rupture of membranes for longer than 4 hours Other events increasing fetal exposure to maternal blood |
HIV infection in infants appears to have two forms. The first form, which is similar to HIV infection in adults, has a prolonged course, with HIV infection progressing to AIDS over eight to 10 years. The second and more aggressive form is characterized by early conversion to AIDS and an increased risk of opportunistic infections and mortality.
Early HIV infection increases the risk of rapid progression to AIDS. Defined as a positive virologic test fewer than 48 hours after delivery, early infection is the result of in utero HIV transmission, as opposed to intrapartum or postpartum transmission.10 In a French study11 of HIV infection in children, newborns with hepatosplenomegaly, lymphadenopathy, fewer than 30 percent CD4+ lymphocytes or a positive HIV DNA polymerase chain reaction (PCR) test within the first week after birth were found to be at increased risk for rapid progression of HIV infection. A retrospective study from the Intravenous Immunoglobulin Prophylaxis Clinical Trial12 found that increased viral loads and decreased CD4+ lymphocyte counts increased the relative risk of mortality in HIV-infected infants.
Antiretroviral Drug Therapy
Zidovudine (Retrovir) prophylaxis is recommended for most infants exposed to HIV in utero to decrease the risk of vertical transmission.5 Beginning eight hours after birth, these neonates should receive zidovudine in a dosage of 2 mg per kg every six hours for at least six weeks.
The landmark study proving the benefits of zidovudine prophylaxis was the Pediatric AIDS Clinical Trials Group Protocol 076 (ACTG 076).13 Maternal zidovudine treatment during pregnancy and labor, and neonatal zidovudine therapy for the first six weeks of life, reduced the relative risk of vertical transmission of HIV by 66 percent (Table 2).13 Mothers and infants who did not receive zidovudine had a 25.5 percent chance of vertical transmission, whereas those who received the antiretroviral drug had an 8.3 percent chance (number needed to treat is 5.8). Ideally, HIV-infected mothers receive zidovudine during pregnancy and labor. Even if the mothers have not received antiretroviral drug therapy, their infants should be given zidovudine, with treatment started before eight hours after birth and continuing for six weeks.
| Maternal zidovudine (Retrovir) during pregnancy: 100 mg orally five times daily from 14 weeks of gestation to delivery* |
| Maternal zidovudine during labor: 2 mg per kg by intravenous load over 1 hour, then 1 mg per kg per hour until delivery |
| Neonatal zidovudine: 2 mg per kg orally every 6 hours from 8 hours after delivery until 6 weeks of age |
Mild anemia is the primary side effect of zidovudine prophylaxis in infants. The anemia is maximal at six weeks of life and resolves spontaneously by 12 weeks without treatment.13 Comorbid illnesses such as Rh or ABO incompatibility can exacerbate the anemia. Follow-up data from the ACTG 076 study have shown no long-term adverse effects or delays in diagnosing infants who are HIV positive if the mothers were treated with zidovudine during pregnancy.14
A recent study15 evaluated the use of nevirapine, a nonnucleoside reverse transcription inhibitor, during labor and after birth. HIV-infected mothers were given a single 200-mg dose of nevirapine orally at the onset of labor; their infants were given 2 mg per kg in a single dose administered orally within 72 hours after delivery. These measures were found to decrease vertical transmission of HIV by 47 percent. However, this study was conducted in Africa (Uganda), where 98 percent of infants are breastfed, and all patients in the study were antiretroviral-naïve.
Given the benefits of nevirapine as shown in the study done in Africa,15 there are recommendations for the use of antiretroviral drugs instead of or in addition to zidovudine, based on several clinical scenarios (Table 3).9 Sub-specialty consultation should be considered for infants of mothers who have more complicated or advanced HIV disease, because these infants may benefit from alterations in the standard ACTG 076 treatment protocol.
Maternal Care Before Delivery
In 1998, an expert panel from the National Institutes of Health16 recommended that pregnant HIV-infected women be managed in the same way as nonpregnant HIV-positive women. In both situations, multiple antiretroviral drugs, including protease inhibitors, are used to reduce viral loads to undetectable levels. However, decisions regarding antiretroviral drug therapy during pregnancy are complex and should be made only after patients have been informed of the benefits and risks.
Current recommendations for maternal and neonatal antiretroviral drug therapy are based on four main clinical scenarios (Table 3).9 Recommendations for mode of delivery to decrease the risk of vertical HIV transmission are also given. Elective cesarean section at 38 weeks of gestation (with a first- or second-trimester ultrasound examination to confirm dates when possible) may decrease the risk of HIV transmission. Surgery carries increased risks for mothers; thus, as with antiretroviral drug therapy, benefits and risks should be discussed to allow an informed decision.
| Clinical scenario | Management recommendations | |
|---|---|---|
| Antiretroviral therapy | ||
| Scenario 1: HIV-infected pregnant woman not previously exposed to antiretroviral drugs | Antiretroviral drug therapy is selected based on the same parameters used in nonpregnant HIV-infected women. The regimen should include orally administered zidovudine (Retrovir) during pregnancy and intravenously administered zidovudine during labor. | |
| Scenario 2: HIV-infected woman receiving antiretroviral drugs during current pregnancy | Continuation of antiretroviral drug therapy should be considered. Zidovudine should be incorporated into the regimen and should be given intravenously during labor. | |
| Scenario 3: HIV-infected woman in labor with no previous antiretroviral drug therapy | Consider one of four regimens: 1. Single dose of orally administered nevirapine (Viramune) given to the mother at the onset of labor, and single dose given to the newborn by 48 hours after birth 2. Orally administered lamivudine-zidovudine (Combivir) given to the mother during labor and to the newborn for 1 week after birth 3. Intravenously administered zidovudine given to the mother during labor, and orally administered zidovudine given to the newborn for 6 weeks after birth 4. Two doses of orally administered nevirapine and intravenously administered zidovudine given to the mother during labor, and orally administered zidovudine given to the newborn for 6 weeks after birth | |
| Scenario 4: Infant of an HIV-infected mother who did not receive antiretroviral drugs during pregnancy or labor | Give orally administered zidovudine to the newborn for 6 weeks after birth. Consider use of additional antiretroviral drugs. | |
| Mode of delivery | ||
| Scenario A: HIV-infected woman presenting late in pregnancy, not receiving antiretroviral drug therapy and unlikely to have laboratory evaluations before delivery | Begin antiretroviral drug therapy. Consider elective cesarean section at 38 weeks of gestation. | |
| Scenario B: HIV-infected woman initiating prenatal care in third trimester, receiving highly active antiretroviral drug therapy but with a viral load of >1,000 copies per mL | Continue antiretroviral drug therapy as long as the viral load is dropping appropriately. Consider elective cesarean section at 38 weeks of gestation. | |
| Scenario C: HIV-infected woman on highly active antiretroviral drug therapy with an undetectable viral load | Whether elective cesarean section has any additional benefit is unclear. The risk of vertical transmission of HIV is less than 2 percent with vaginal delivery. | |
| Scenario D: HIV-infected woman who has elected cesarean section for delivery but presents in labor | Begin intravenous administration of zidovudine. If delivery is imminent, vaginal delivery may be used, with oxytocin (Pitocin) augmentation considered. If a long labor is anticipated, consider proceeding with cesarean section. The risk of vertical transmission of HIV is increased with rupture of membranes for longer than 4 hours. | |
Monitoring of Newborns
Early detection of HIV infection is important. Before 1994, the only widely available tests for HIV were an enzyme-linked immunosorbent assay (ELISA) and a Western blot test that detected HIV-specific IgG. Reliance on these tests delayed definitive diagnosis of HIV infection in infants because maternal IgG antibody crosses the placenta and remains detectable in the newborn's blood up to 18 months.6
A number of tests that directly detect HIV are now available. Compared with antibody-dependent tests, the HIV DNA PCR test, HIV culture, p24 core antigen level, and HIV RNA viral level all allow earlier detection of neonatal HIV infection. The HIV DNA PCR test is the recommended initial screening tool in infants born to HIV-positive mothers. The test has a sensitivity of 93.2 percent and a specificity of 94.9 percent; however, it is less accurate in neonates. In infants with a low risk of transmission, the positive predictive value of the HIV DNA PCR test is 55.8 percent during the first month after birth and 83.2 percent after the first month.17
Although the HIV culture is as sensitive as the HIV DNA PCR test, culture is more expensive, and results are not available for two weeks.18 Of the direct detection tests, the p24 antigen level is the least sensitive and has a high false-positive rate.19–21 The HIV RNA viral level is a useful measure of disease progression in patients with known HIV infection. However, the HIV RNA viral level is generally not recommended for use in screening infants for HIV infection, because commercially available assays have a lower sensitivity and specificity than the HIV DNA test.22
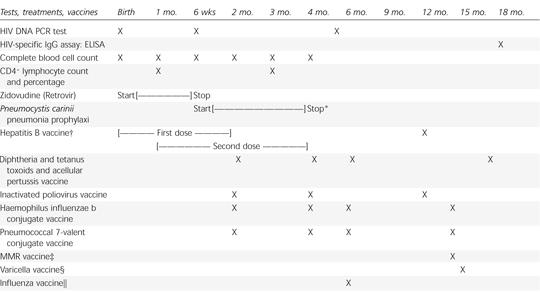
The HIV DNA PCR test should be performed before the newborn of an HIV-positive mother is 48 hours old. The test should be repeated when the infant is one to two months old, and again at age four to six months6 (Table 4).4,7,23 The first PCR test should not be performed on cord blood because of an increased risk for a false-positive result. HIV infection is definitively diagnosed by two positive virologic tests performed on separate blood samples.
If three virologic tests are negative (at birth, one month of age and four months of age), there is a 95 percent chance that the infant is not infected with HIV.7 A negative HIV-specific IgG assay (ELISA) at 18 months of age definitively rules out HIV infection in exposed infants.
The CD4+ lymphocyte level should be monitored to assess the infant's immune status, and the complete blood cell count should be followed to monitor for medication side effects. The absolute number of CD4+ lymphocytes varies with age, but the percentage of CD4+ lymphocytes does not; hence, the CD4+ percentage is a better marker for assessing an infant's immune status.
Pneumocystis carinii Pneumonia Prophylaxis
Pneumocystis carinii pneumonia in infants can have an acute onset and a high mortality rate. It is often the first indicator of perinatal HIV infection.24 In HIV-infected infants, the peak incidence of P. carinii pneumonia is at three to six months of age. This pneumonia can occur in HIV-infected children younger than one year, regardless of the CD4+ lymphocyte count (unlike in HIV-infected adults). Therefore, all newborns of HIV-infected mothers should receive P. carinii pneumonia prophylaxis starting at six weeks of age and continuing until HIV infection is excluded.25
The recommended agent for P. carinii pneumonia prophylaxis is trimethoprim-sulfamethoxazole (Bactrim, Septra).26 Dapsone and atovaquone (Mepron) are possible alternatives (Table 5).25 Because of the side effects of these medications, the complete blood count should be evaluated at the initiation of therapy and monthly thereafter.16
| Recommended drug: trimethoprim-sulfamethoxazole suspension (Bactrim, Septra). | |
| All dosing regimens use trimethoprim at 150 mg per m2 daily (or 5 mg per kg daily) with sulfamethoxazole at 750 mg per m2 daily (or 25 mg per kg daily) | |
| Recommended dosing regimen: trimethoprim-sulfamethoxazole taken orally twice daily three times a week on three consecutive days (e.g., Monday, Tuesday, Wednesday) | |
| Acceptable alternative dosing regimens for trimethoprim-sulfamethoxazole: | |
| Orally once daily three times a week on three consecutive days | |
| Orally twice daily for one week | |
| Orally twice daily three times a week on alternate days (e.g., Monday, Wednesday, Friday) | |
| Alternative drugs and regimens*: | |
| Dapsone: in infants 1 month of age and older, the dosage is 2 mg per kg taken orally once daily (maximum of 100 mg per dose) | |
| Atovaquone (Mepron): in infants 1 to 3 months of age and young children older than 24 months of age, the dosage is 30 mg per kg daily taken orally; in infants and young children 4 to 24 months of age, the dosage is 45 mg per kg daily taken orally. | |
P. carinii pneumonia prophylaxis should be started after completion of six weeks of zidovudine therapy. Prophylaxis is not recommended before four weeks of age because of the low incidence of this pneumonia in neonates. Also, trimethoprim-sulfamethoxazole can exacerbate the anemia caused by zidovudine and increase adverse effects on the newborn's immature bilirubin metabolism.
Prophylactic medication can be discontinued when two HIV DNA PCR tests are negative (one after the infant is one month old and the other after the infant is four months old). Prophylaxis in an HIV-infected infant should be continued until the age of 12 months, regardless of the CD4+ lymphocyte count. After the age of 12 months, the need for prophylaxis is determined by the CD4+ lymphocyte count.
Screening for Tuberculosis
The reemergence of tuberculosis has coincided with the spread of HIV.27 Infants infected with HIV and all children living with an HIV-positive person are at increased risk for tuberculosis. HIV-infected pregnant women should be screened for tuberculosis before delivery. Infants should be kept away from any person with active pulmonary disease until that person is no longer considered to be contagious.7
Infants and young children exposed to a person with active tuberculosis should have a purified protein derivative (PPD) skin test and a chest radiograph. A positive PPD test in HIV-exposed or HIV-infected children is 5 mm of induration. Even if the PPD test is negative, infants who have been exposed to tuberculosis should be given isoniazid (INH) for three months.7 Then, the PPD test should be repeated. If the test is negative, isoniazid may be discontinued. If the test is positive, prophylaxis is continued.28 All HIV-infected children should have a screening PPD test annually, starting at 12 months of age.
Modifications of Standard Infant Care
Although perinatal exposure to HIV generally does not result in prematurity or low birth weight, newborns need to be monitored closely for appropriate growth and neurodevelopment, as well as signs of perinatal exposure to other infectious diseases.29
The HIV-exposed newborn should be tested for syphilis, hepatitis B, and hepatitis C if the mother has not been screened. If the mother is hepatitis B surface antigen–positive, the infant should receive hepatitis B immunoglobulin and hepatitis B vaccine at birth, and hepatitis vaccine again at one to two months of age and six months of age.
Live virus vaccines should be used with caution in HIV-exposed infants, both for their safety and the safety of HIV-infected family members. The combination measles-mumps-rubella vaccine can be given to the HIV-positive infant who has only a minimally to moderately compromised immune system (Centers for Disease Control and Prevention [CDC] immune category I or II).23 Varicella vaccine can be given to the HIV-positive infant who has a minimally compromised immune system (CDC immune category I) after parents have been informed about the benefits and risks of immunization.23 Influenza vaccine should be given annually starting at six months of age until HIV infection has been ruled out.29
Immunizations may not be effective in immunocompromised persons. The ability to respond to vaccines is likely to be related to overall immune status at the time of immunization. As a result, infants of HIV-infected mothers who are exposed to vaccine-preventable diseases (e.g., varicella, measles, tetanus) should be considered for passive immunoprophylaxis or chemoprophylaxis, regardless of immunization status, unless recent serologic tests demonstrate adequate antibody concentrations.23
At each routine examination, parents and other caregivers should be educated about warning signs of HIV and opportunistic infections. HIV-infected infants are at increased risk for serious bacterial infections. Fever warrants early evaluation and presumptive treatment, pending further clarification of its cause.
Parents and other caregivers also should be educated about preventing exposure to opportunistic infections. Appropriate measures include avoiding undercooked foods (salmonellosis), cat litter boxes (toxoplasmosis), and potentially contaminated water (giardiasis and cryptosporidiosis).7
Psychosocial Issues
HIV-infected mothers may have multiple physical, emotional, and social concerns, including coming to terms with the reality of their own infection while facing uncertainty about the HIV status of their infant. Physicians should help these women obtain necessary social services, access to appropriate sub-specialist care, and guidance in providing long-term care for their children in the event of their incapacitation or death.