
Am Fam Physician. 2002;65(11):2263-2272
Family physicians play a key role in assessing and managing patients with Alzheimer's disease and in linking the families of these patients to supportive services within the community. As part of comprehensive management, the family physician may be responsible for coordinating assessments of patient function, cognition, comorbid medical conditions, disorders of mood and emotion, and caregiver status. Suggestions for easily administered and scored assessment tools are provided, and practical tips are given for supporting primary caregivers, thereby increasing efficiency and quality of care for patients with Alzheimer's disease.
Advances have been made in the clinical diagnosis and treatment of Alzheimer's disease. Using the criteria established by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association,3 autopsy results support the clinical diagnosis in 86 to 90 percent of cases.4 Advances in therapy include the introduction of cholinesterase inhibitors5 and the use of antioxidants.6
| Assessment | ||
| Conduct and document assessments of the following: | ||
| Daily function, including feeding, bathing, dressing, mobility, toileting, continence, and ability to manage finances and medications | ||
| Cognitive status, using a reliable and valid instrument such as the Mini-Mental State Examination | ||
| Other medical conditions | ||
| Behavioral problems, psychotic symptoms, and depression | ||
| Reassess the patient every 6 months or more frequently if indicated. | ||
| Identify the primary caregiver and assess the adequacy of family and other support systems. | ||
| Assess the culture, values, primary language, and decision-making process of the patient and family. | ||
| Treatment | ||
| Develop and implement an ongoing treatment plan, with defined goals that include the following: | ||
| Use of cholinesterase inhibitors, if clinically indicated, to treat cognitive decline | ||
| Referral for appropriate structured activities such as exercise, recreation, and adult day care | ||
| Appropriate treatment of comorbid medical conditions | ||
| Treat behavioral problems and mood disorders using the following: | ||
| Nonpharmacologic approaches such as environmental modification, task simplification, and appropriate activities | ||
| Referral to social service agencies or support organizations, including the Alzheimer's Association Safe Return Program for people who wander | ||
| Medications, if clinically indicated | ||
| Patient and caregiver education and support | ||
| Discuss the diagnosis and progression of Alzheimer's disease with the patient and family in a manner consistent with their values and preferences, as well as the patient's abilities. | ||
| Refer the patient and family to organizations that can provide educational materials on community resources, support groups, legal and financial issues, respite care, and future care needs and options. Sources of information and support include the following: | ||
| Local social services department | ||
| Alzheimer's Association: national telephone: 800-272-3900; Web site:www.alz.org | ||
| Family Caregiver Alliance: national telephone: 800-445-8106; Web site:www.caregiver.org | ||
| Alzheimer's Disease Education and Referral Center: national telephone: 800-438-4380; Web site:www.alzheimers.org/adear | ||
| Discuss the patient's need to have advance directives and to identify surrogates for medical and legal decision-making. | ||
| Reporting requirements | ||
| Monitor for evidence of abuse. Report all instances of abuse to the local police department and/or social services department as required by local law. | ||
| Report the diagnosis of Alzheimer's disease to the appropriate motor vehicle or other department in accordance with local law. | ||
Several professional organizations and work groups have developed consensus statements and guidelines for the diagnosis and treatment of Alzheimer's disease from the perspective of subspecialty care.7–9 To date, however, user-friendly, practical guidelines have not been available for busy family physicians. Recent studies have shown that many family physicians have relatively limited knowledge of dementia.10
The growth of managed care as the principal means of health care delivery in the United States has increased demands on family physicians. In addition, the growth of managed Medicare has increased the number of elderly persons receiving medical care from family physicians. This article offers practical guidance and tools to help family physicians provide a comprehensive management program for patients with Alzheimer's disease.
The management guidelines discussed in this two-part article and summarized in Table 1 are intended to be used in directing the care of patients and their caregivers after the diagnosis of Alzheimer's disease has been made. They are most relevant to the management of community-dwelling patients being cared for by family members. The guidelines do not address the initial identification, evaluation, or differential diagnosis of memory complaints in elderly patients; these issues are covered in other guidelines. Part I of this two-part article focuses on assessment, and part II reviews treatment.
Assessment
The five areas that require assessment (and periodic reassessment) in the patient with Alzheimer's disease are daily function, cognition, comorbid medical conditions, disorders of mood and emotion, and caregiver status.
DAILY FUNCTION
An assessment of daily function is critical to an understanding of the degree of the patient's disability and dependence on the caregiver. Basic activities of daily living (ADL), such as feeding and toileting, can be assessed with an interview or by using a tool such as the ADL scale (Figure 1).11 The results of the assessment enable realistic planning for necessary supportive interventions.
The ADL assessment form is used to evaluate the degree of assistance received by the patient during a set period (e.g., the previous week) for each of six activities: bathing, dressing, toileting, transfer, continence, and feeding. The family physician or an assigned rater (nurse, caregiver, etc.) records the actual assistance given, not the capability of the patient.
Data recorded on the ADL evaluation form are converted to an ADL point scale ranging from zero (complete independence in all six categories) to 6 (complete dependence in all six functions). Intermediate scores of 1 to 5 indicate various degrees of independence. For a patient to be considered dependent, the third column of the ADL form must be checked. The ADL instrument can be repeated to determine changing needs for assistance.
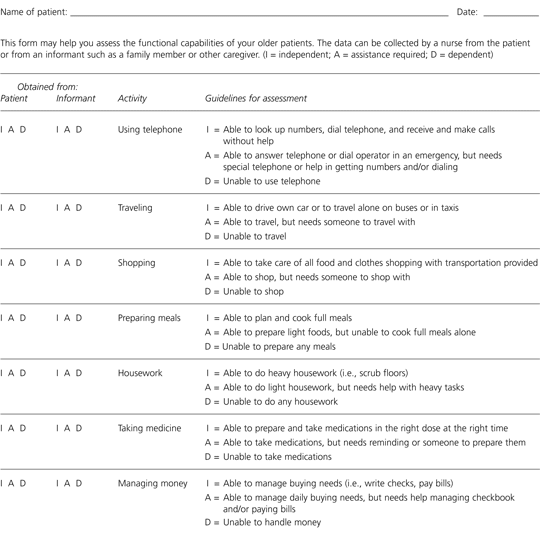
Instrumental activities of daily living (IADLs), such as shopping, cooking, and managing finances and medications, can also be assessed using formal instruments or informal questioning of the primary caregiver. The IADL scale is most frequently used. This instrument measures seven areas of more complex activities required for optimal independent functioning (Figure 2).12 The scoring indicates whether the patient is completely independent, requires assistance, or is dependent for the performance of each activity. As with the ADL scale, the IADL instrument may be repeated periodically to determine the need for more support.
An in-home assessment may identify environmental supports needed to maximize the patient's function, ensure the patient's safety, and minimize the primary caregiver's distress.
COGNITION
Cognitive status should be reassessed periodically during the course of Alzheimer's disease. The timing of reassessments depends on the stage of the illness and the monitoring required for comorbid medical conditions. Based on consensus discussions during the development of the guidelines presented in this article, reassessment every six months is recommended as a general rule.
Optimal management requires an understanding of disturbances of memory, language, and visuospatial skills. Although brief cognitive tests are available for assessing dementia, one survey13 found that approximately 40 percent of physicians did not use formal mental status testing in elderly patients.
The annotated Mini-Mental State Examination (MMSE) is the brief instrument most commonly used to assess cognitive changes in patients with dementia.14 The MMSE covers six areas: (1) orientation, (2) registration, (3) attention and calculation, (4) recall, (5) language, and (6) ability to copy a figure. This easily administered 30-item instrument screens for cognitive deficits, aids in the diagnosis of dementia, and can be used serially to quantify changes in cognitive function. The MMSE has been translated into many languages and, with modest adjustments, can be used in a variety of cultural settings.
A perfect score on the MMSE is 30 points. A total score of 23 or less suggests dementia. However, MMSE scores indicative of cognitive impairment vary by age and education; normative values are available for these adjustments.15
The MMSE has limitations. The instrument is not sensitive in detecting mild dementia; abnormalities are not specific for Alzheimer's disease or dementia. Furthermore, late in the course of Alzheimer's disease, the test has a “floor effect” (with patients scoring at the bottom of the range despite worsening dementia). On average, the MMSE score changes at a rate of approximately three or four points per year in patients with Alzheimer's disease. More marked worsening should trigger a search for complicating comorbid illness or another dementing illness.
A one-time referral for formal neuropsychologic testing may be helpful in distinguishing Alzheimer's disease from the normal effects of aging and in characterizing the deficits present on initial assessment.
COMORBID MEDICAL CONDITIONS
Patients with Alzheimer's disease frequently have comorbid medical conditions such as cardiovascular disease, infection, pulmonary disease, renal insufficiency, arthritis, and diminution of vision and hearing. Appropriate treatment of these conditions can optimize patient function and minimize excess disability.
The approach to managing cormorbid medical conditions must take into account the stage of dementia and its effects on care planning, communication methods, benefits and risks of treatments, and adherence to treatment. The family physician must evaluate the patient's capacity to participate in treatment decisions and, as necessary, involve the caregiver in helping make informed choices.
In the early stage of Alzheimer's disease, the patient may be lucid. During each medical visit, however, the patient's capacity to understand and communicate must be reassessed before information and instructions are provided. Written instructions and reminders are helpful in increasing adherence to and correct implementation of the physician's recommendations. The caregiver can be a valuable ally in ensuring that medications are taken and other treatment activities are accomplished correctly. Potential problems may be identified earlier if the caregiver is coached to watch for behavioral changes that may indicate adverse treatment effects.
The primary caregiver and other family members may assess treatment options differently when a patient is in the late stage of Alzheimer's disease, because of diminished treatment benefit in the context of dementia and quality-of-life issues. In the patient with advanced disease, management is directed at infectious illnesses, nutritional and feeding difficulties, bowel and urinary disorders, mobility-associated problems, and pressure ulcerations.16
DISORDERS OF MOOD AND EMOTION
The patient with Alzheimer's disease should be assessed periodically for behavioral problems, psychotic symptoms, and depression. Behavioral problems eventually occur in nearly all patients with the disease.17 These problems are a major cause of caregiver distress and one of the principal determinants of institutionalization. Behavioral disturbances may be directly observed by the physician but are more often reported by the primary caregiver.
The patient should be evaluated for drug toxicity and medical, psychiatric, psychosocial, or environmental problems that may underlie behavioral changes. In general, reassessment every six months is necessary because new behaviors emerge over the course of Alzheimer's disease. The care plan should include potentially useful nonpharmacologic interventions as well as adequate precautions to reduce the risk of harm to the patient and others, to minimize excess disability associated with treatable behavioral or mood disturbances, and to reduce the risk of residential placement.
Agitation
This behavioral disturbance is common in patients with Alzheimer's disease.17 Agitation can have a number of triggers, including pain, medications, and psychosocial stressors. A thorough assessment is essential to rule out iatrogenesis and treatable contributing causes.
Psychosis
Psychotic symptoms are less common than agitation but increase in frequency as Alzheimer's disease progresses.17 Several rating scales are available to assess agitation, psychosis, and other types of behavioral disturbances.
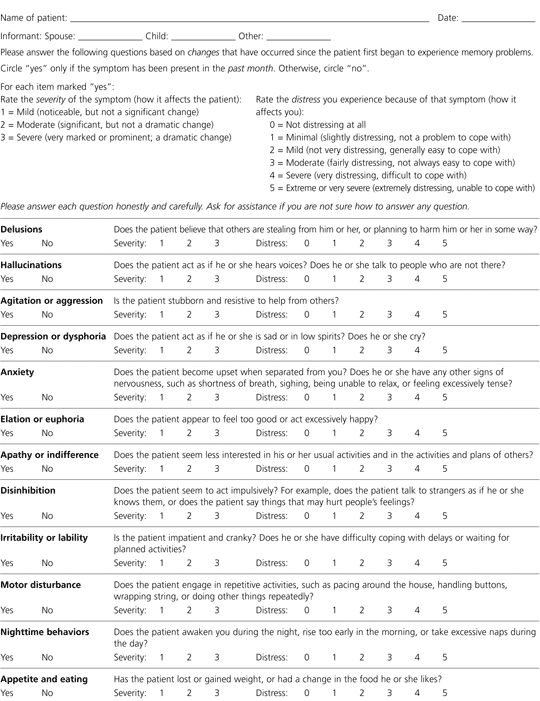
The Neuropsychiatric Inventory Questionnaire (NPI-Q) is a rapidly administered instrument that provides a reliable assessment of behaviors commonly observed in patients with dementia (Figure 3).18 The NPI-Q may be a useful tool for family physicians because it assesses the severity of the symptom in the patient and the distress the symptom causes in the caregiver.
Depression
The Geriatric Depression Scale (GDS) is a 30-item, self-rated or observer-rated screening instrument for use in cognitively intact older adults.19 A score between zero and 10 is considered to be in the normal range; a score of 11 or higher may indicate the presence of depression and warrants a more thorough evaluation. GDS scores may underreport depression in patients with mild to moderate Alzheimer's disease.20
CAREGIVER STATUS
The physical and emotional health of the primary caregiver is critical to optimal care of the patient with Alzheimer's disease. Caregivers suffer from increased rates of depression and physical illness and are prescribed medications at a higher rate than persons not required to be in a care-giving role.21
Assessment of caregiver status can lead to the implementation of measures that minimize patient-caregiver stress and defer institutionalization of the patient. The family physician should conduct the assessment or refer the caregiver to a psychologist, social worker, or other member of the health care delivery team.
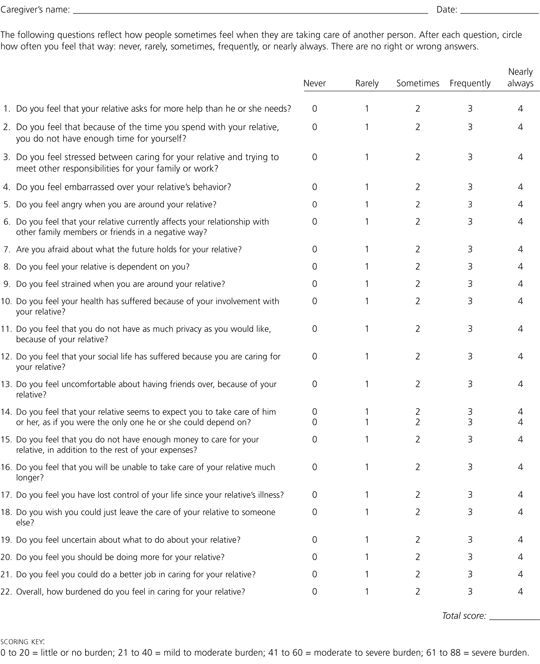
Various tools have been developed to provide information about the activities and concerns of persons who care for patients with Alzheimer's disease. One of the earliest and most widely used tool is the Caregiver Burden Scale, a self-administered 22-item questionnaire with a five-item response set ranging from “never” to “nearly always” (Figure 4).22 The numbers for the responses are added to obtain the total score, with higher scores indicating greater caregiver distress.
Special Factors in Caregivers and Patients
Members of ethnic minority groups have different care-giving patterns than persons in the majority culture, and they may place different interpretations on memory and behavioral problems. Black and Hispanic families, for example, distribute care among multiple family members, rather than having one primary caregiver as occurs in most white families. In addition, decision-making processes vary among families; sensitivity to the nuances of family decision-making is required to establish an effective working relationship with family caregivers.
Reassessment
Regular reassessments are critical to all aspects of the management of patients with Alzheimer's disease. The frequency of reassessment depends on the acuity of the needs of the patient and caregiver. It is generally necessary to see the patient every six months during the course of the illness, and more frequently when complex or potentially dangerous symptoms emerge or when new drug therapies are being introduced. These regular visits also allow reassessment of the caregiver, identification of caregiver burnout, referral of the caregiver to support groups, and the initiation of other appropriate interventions.