
Am Fam Physician. 2002;66(1):107-113
Growing evidence shows that early detection of cancer can substantially reduce mortality, necessitating screening programs that encourage patient compliance. Radiology is already established as a screening tool, as in mammography for breast cancer and ultrasonography for congenital anomalies. Advanced processing of helical computed tomographic data sets permits three-dimensional and virtual endoscopic models. Such models are noninvasive and require minimal patient preparation, making them ideal for screening. Virtual endoscopy has been used to evaluate the colon, bronchi, stomach, blood vessels, bladder, kidney, larynx, and paranasal sinuses. The most promising role for virtual endoscopy is in screening patients for colorectal cancer. The technique has also been used to evaluate the tracheobronchial tree for bronchogenic carcinoma. Three-dimensional and virtual endoscopy can screen, diagnose, evaluate and assist determination of surgical approach, and provide surveillance of certain malignancies.
Recent advances in imaging technology allow three-dimensional and virtual endoscopic models to be constructed from helical computed tomographic (CT) data sets. Helical, or spiral, CT scanning permits continuous imaging as the radiographic tube rotates around the moving patient, whereas conventional CT scanning is limited to a series of 360-degree slices through the stationary patient. A conventional CT scan can be conceptualized as a stack of slices, whereas a helical scan is a continuous helix (similar to a child's Slinky® toy). Helical CT scanning offers several advantages. It is faster than the conventional technique and provides more information in the craniocaudal axis. It yields continuous data with less respiratory or bowel motion misregistration.
Virtual endoscopy and perspective volume rendering are constructed using data obtained from helical CT scanning and commercially available software, opening the way for exciting new clinical applications. Two methods exist for postprocessing of data: surface rendering and volume rendering. Surface rendering links the contours of selected objects in a given slice with adjacent slices. This is a faster processing system but subject to poor definition, data loss, and threshold artifacts.1 True perspective volume rendering allows for more detail and opacity but entails increased processing time and cost.
These techniques are being studied at research centers for use in a variety of clinical applications, including inspection of the colon, tracheobronchial tree, blood vessels, urinary tract, facial bones, and sinuses. Although not yet in routine use, the techniques have been found useful in specific scenarios, such as virtual colonoscopy and virtual bronchoscopy. Some centers no longer consider virtual colonoscopy a research protocol and are offering it as a screening tool despite its limitations.
With continuing advances in software and hardware, virtual endoscopy offers the promise of quicker and cheaper methods of evaluation. In certain clinical situations, virtual endoscopy may enhance diagnosis, pre-operative planning, operative technique, and postoperative follow-up.
Primary care physicians will become more familiar with this new imaging method as more clinical applications become apparent.
Virtual Colonoscopy
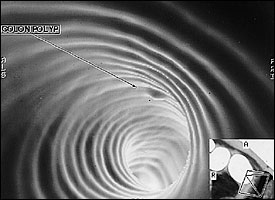
Early detection of colon cancer is the key to a good prognosis. The five-year survival rate for colon cancer is nearly 90 percent for localized disease versus about 6 percent for distant metastases.3 It can take from 10 to 15 years for an adenomatous polyp to become an invasive cancer.4 Thus, there is a considerable time for detection and clinical intervention if the proper screening methods are used. Studies of fecal occult blood testing and flexible sigmoidoscopy have shown that screening for colorectal cancer in high-risk countries can decrease mortality by 50 percent.5 Yet, it is reported that fewer than 20 percent of American adults have undergone colorectal cancer screening.6
CONVENTIONAL SCREENING
Conventional screening examinations for colorectal cancer are eligible for Medicare reimbursement. The American Cancer Society guidelines for screening are outlined in Table 1.7 A similar set of guidelines was established by the Agency for Health Care Policy and Research (now called the Agency for Healthcare Research and Quality).8 The choice of procedure should depend on medical status and community-specific quality of available examination methods. Screening is a more complex issue in moderate- to high-risk patients (those with a history of colorectal cancer, adenomatous polyps, inflammatory bowel disease, familial adenomatous polyposis, or family history of colorectal cancer).
| Beginning at age 50, persons at average risk should follow one of the three screening options below: | |
| Annual fecal occult blood test plus flexible sigmoidoscopy every five years* | |
| Colonoscopy every 10 years* | |
| Double-contrast barium enema every five years* | |
Each of the conventional screening procedures has specific disadvantages. The fecal occult blood test is not useful in detecting a precancerous stage because large adenomatous polyps rarely cause bleeding and remain undetected.9 In fact, the test's reported sensitivity varies from 38 percent to 92 percent and is extremely laboratory-dependent.8 Flexible sigmoidoscopy has a restricted range and cannot go beyond the splenic flexure.
A recent study to determine the prevalence and location of advanced colonic neoplasms found that 52 percent of 128 patients with proximal neoplasms had no distal adenomas.10 These neoplasms would not have been detected with examination of the distal colon or flexible sigmoidoscopy. Therefore, an adequate screening method should visualize the entire colon. In fact, in one quarter of patients with precancerous polyps or invasive cancer, sigmoidoscopy and the fecal occult blood test will miss the lesions when performed once, in combination.11
Colonoscopy can, in most cases, visualize the entire colon and is the “gold standard” of examination methods. However, the procedure does not permit passage through obstructions or twisted portions of bowel. Furthermore, the United States Congress Office of Technology Assessment reported that colonoscopy is associated with the risk of bowel perforation (1:1,000 procedures) and mortality (1:5,000 procedures).12 Finally, colonoscopy is invasive and time-consuming, and these disadvantages can result in decreased patient compliance.
Virtual colonoscopy provides several advantages as a screening tool (Table 2). It allows the examiner to visualize the entire colon and adjacent anatomic structures. The procedure also allows visualization of areas distal to an obstructed or twisted bowel, providing information on occlusive carcinomas. In addition, virtual colonoscopy can be a valuable tool in preoperative evaluation of the colon.15 It defines the exact anatomic location of abnormalities and the proximity of adjacent structures, whereas conventional colonoscopy only estimates the location of lesions.
| Advantages | Disadvantages |
|---|---|
| Noninvasive No sedation required Can image entire colon and localize lesions precisely Fast Sensitivity equal to that of colonoscopy for lesions >10 mm in diameter and superior to that of DCBE Less technically demanding | Cost* Radiation exposure Does not allow biopsy specimens to be taken Cannot visualize polyps <1 cm in diameter Cannot show texture and color details of mucosa Retained feces can be misinterpreted as polyps |
Virtual colonoscopy rivals conventional colonoscopy and exceeds double-contrast barium enema in sensitivity for colorectal polyps.13,16,17 Virtual colonoscopy's sensitivity is related to polyp size.10,18,19 Sensitivity and specificity have been reported as 75 percent and 90 percent, respectively, for polyps greater than 10 mm in diameter.18 Sensitivity drops for smaller polyps (less than 10 mm); however, these are also much less likely to develop into cancer. At present, the size designating a clinically significant polyp is a matter of controversy. The sensitivity of virtual colonoscopy is actually higher for adenomatous polyps, the main precursor to colorectal cancer, than for hyperplastic polyps.18 False-negative results can occur with flat lesions and inadequate insufflation, while false-positive results are often due to residual feces.1
Certain aspects of virtual colonoscopy can lead to increased patient compliance. Virtual colonoscopy is faster than colonoscopy. It is minimally invasive and does not involve sedation or significant loss of work time. Patient preparation requires only breath holding, a colon cleansing preparation and air insufflation, with or without intravenous smooth muscle relaxant. Of 100 patients who underwent virtual colonoscopy, none requested the examiner to stop because of discomfort or pain.18
Actual costs for the routine use of virtual colonoscopy as a screening tool are not available. To become cost-effective and enter widespread use, virtual colonoscopy would have to be inexpensive and demonstrate high patient compliance compared with conventional colonoscopy.20 At present, there is no specific reimbursement code for virtual colonoscopy. To achieve cost-effectiveness as a screening test, the procedure will require a specific reimbursement code that will differentiate it from abdominal and pelvic CT scanning. Virtual colonoscopy is not now eligible for Medicare reimbursement; however, it is being offered in many communities and academic centers.
The radiation exposure incurred during a virtual colonoscopy examination is currently equivalent to that of two plain abdominal films and will probably decrease with continued software and hardware developments.19 Reports have shown that by using low-current radiation, the total dose of radiation from virtual colonoscopy is less than that incurred in a double-contrast barium enema.1 Because retained fecal matter can be misinterpreted as polyps, one alternative is to use specific contrast material to label fecal matter. Virtual colonoscopy is somewhat limited in visualizing mucosal detail and polyps smaller than 1 cm,23 and it does not allow for biopsy specimens to be taken.
A consensus will need to be reached on the size of polyps that should be biopsied. If a screening examination using virtual colonoscopy shows a polyp or polyps that meet the designated criterion, the next step would likely be conventional colonoscopy with polypectomy. Despite its obvious limitations, virtual colonoscopy could lead to increased patient compliance and cancer detection, resulting in less colon cancer.
Virtual Bronchoscopy
The work-up for chest tumors and metastatic disease routinely includes a helical CT scan and bronchoscopy to evaluate patients preoperatively and postoperatively. Bronchoscopy allows the physician to determine the extent of the disease. Unfortunately, this procedure has some disadvantages. It is invasive, requires sedation and cannot be tolerated by some patients, especially children. In addition, the bronchoscope cannot pass areas of stenosis or occlusion, prohibiting evaluation of distal areas.24
Virtual bronchoscopy simulates an actual bronchoscopic examination by computing data acquired from helical CT scans (Figure 2). Virtual bronchoscopy can visualize beyond the segmental bronchus, allowing the physician to detect stenosis, occlusion, and external impression on the bronchial lumen.24 In addition, CT images obtained during virtual bronchoscopy provide bronchoscopic views not only of the airways but also of lung parenchyma.
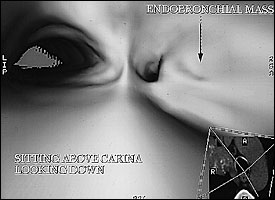
Studies comparing virtual to fiberoptic bronchoscopy have shown that although there are “no significant differences in grading [the degree] of stenosis,”24 virtual bronchoscopy has certain advantages (Table 3). Virtual bronchoscopy is a noninvasive procedure that requires only breath holding as patient preparation.1 Virtual bronchoscopy can pass high-grade stenoses and allows examination of distal (peripheral) airways.24 An added benefit is that virtual bronchoscopy can also anatomically localize lesions.1 Airway stenoses may be reliably evaluated with virtual bronchoscopy to the level of the segmented bronchus.25 Automated navigational aids and detection software are being developed and refined.26 In addition, the position of extrabronchial processes, such as external impressions, can be correlated with cross-sectional images, aiding in localization.24 Furthermore, virtual bronchoscopy can be an excellent teaching tool, in that the clinician can simulate the procedure before the actual bronchoscopy.
| Advantages | Disadvantages |
|---|---|
| Noninvasive No sedation required Can image airways distal to high-grade stenosis Can localize parenchymal lesions Teaching tool | Does not allow biopsy specimens to be taken Cannot show texture and color details of mucosa Has difficulty visualizing areas of viscous secretions (e.g., blood) |
Virtual bronchoscopy's disadvantages are its inability to obtain biopsy material and show color, friability and detail of the mucosal surface.24 Distal portions of the bronchial tree can become difficult to assess if they are filled with viscous secretions, such as blood.24 However, the combination of virtual bronchoscopy and crosssectional imaging could improve preoperative, intraoperative and postoperative evaluations. Precise localization of anatomic structures and abnormalities with virtual bronchoscopy could be helpful in the selection of a biopsy site and an operative approach, and in follow-up. Sensitivity and specificity of virtual bronchoscopy have not been determined.
Other Applications
The same models and software discussed in this article have been used in other areas of medicine. For example, vascular applications can be valuable in the assessment of abdominal or cerebral aneurysms, carotid or renal stenoses, and atherosclerotic plaques. Three-dimensional CT angiography may assist in the evaluation of cerebral aneurysms and could potentially obviate conventional invasive angiography in some cases. Virtual angioscopy can aid in the characterization of broad-based aneurysms, which can help to determine whether surgical treatment is preferable to coil embolization.27
Virtual endoscopy has also been used to evaluate the bladder, kidneys, small intestine, stomach, larynx, nasolacrimal ducts and paranasal sinuses. Therefore, this method could potentially provide a means for the screening and surveillance of bladder tumors, which tend to recur (Figure 3).

Virtual endoscopy can also be used to simulate endoscopic surgery before the actual performance, thus helping the surgeon plan the operative approach. In summary, virtual endoscopy is a nascent technique with multiple potential applications that could have a significant impact on common clinical issues, especially colorectal cancer screening. Improved screening could detect certain cancers at an early, curable stage and could prevent the development of cancer.