
Am Fam Physician. 2002;66(4):601-609
Unruptured intracranial aneurysms occur in up to 6 percent of the general population. Most persons with these aneurysms remain asymptomatic and are usually unaware of their presence. Risk factors for the formation of aneurysms include a family history of aneurysm, various inherited disorders, age greater than 50 years, female gender, current cigarette smoking, and cocaine use. Because of the morbidity and mortality associated with surgical intervention, screening for aneurysms remains controversial. Two groups of patients may benefit from early detection: those with autosomal dominant polycystic kidney disease and those with a history of aneurysmal subarachnoid hemorrhage. These patients should undergo magnetic resonance angiography, followed by neurosurgical referral if an aneurysm is detected. Screening of patients who have two or more family members with intracranial aneurysms is controversial. Screening of patients who have one first-degree relative with an aneurysm does not appear to be beneficial.
Intracranial aneurysm is a fairly common condition that is often asymptomatic until the time of rupture. Subarachnoid hemorrhage associated with aneurysmal rupture is a potentially lethal event with a mortality rate as high as 50 percent.1 Many patients who survive the initial hemorrhage have permanent disability. Recent research has provided a greater understanding of intracranial aneurysms.
Epidemiology and Risk Factors
An aneurysm is an abnormal localized dilation of any vessel. Because of certain histopathologic and hemodynamic factors, aneurysms most commonly occur in arteries that supply blood to the brain. A recent systematic review2 of studies involving more than 56,000 patients found that unruptured intracranial aneurysms occur in 3.6 to 6 percent of the general population.
Inherited and acquired risk factors have been associated with the formation of intracranial aneurysms (Table 1).3 Familial clustering of these aneurysms may occur with no other history of hereditary disease. The incidence of intracranial aneurysms is between 8 and 9 percent in persons with two or more relatives who have had a subarachnoid hemorrhage or an aneurysm.4,5 Compared with other family members, the siblings of affected persons have a higher risk of developing aneurysmal subarachnoid hemorrhage.6
| Inherited risk factors | Other risk factors |
| Autosomal dominant polycystic kidnesy disease Type IV Ehlers-Danlos syndrome Pseudoxanthoma elasticum Hereditary hemorrhagic telangiectasia Neurofibromatosis type 1 Alpha1-antitrypsin deficiency Coarctation of the aorta Fibromuscular dysplasia Pheochromocytoma Klinefelter's syndrome Tuberous sclerosis Noonan's syndrome Alpha-glucosidase deficiency | Age over 50 years Female gender Current cigarette smoking Cocaine use Infection of vessel wall Head trauma Intracranial neoplasm or neoplastic emboli Hypertension* Alcohol* Oral contraceptive pill use* Hypercholesterolemia* |
Various hereditary connective tissue disorders have been associated with the formation of aneurysms, presumably as a result of the weakening of vascular walls. One study7 found that intracranial aneurysms may develop in 10 to 15 percent of patients with polycystic kidney disease, an autosomal dominant condition. Although Marfan syndrome was previously identified as a risk factor for aneurysms, a recent detailed study8 found no significant relationship. Coarctation of the aorta, fibromuscular dysplasia, and pheochromocytoma have been associated with intracranial aneurysms, most likely because of the elevated blood pressures that occur in these conditions.3
Recent data have shown that age over 50 years, female gender, and current cigarette smoking are risk factors for intracranial aneurysm.9 Since 1984, cocaine use has been linked to the formation and rupture of aneurysms. This association is thought to be due to increased turbulence of blood flow and repeated, transient bouts of hypertension. Among cocaine users, aneurysms have been found in significantly younger patients and in vessels with a smaller diameter.10 Infections from bacterial or fungal colonization of vessel walls, head trauma, and intracranial neoplasms or neoplastic emboli are rare causes of intracranial aneurysms.
Pathophysiology
Intracranial aneurysms are classified as saccular, fusiform, or dissecting. Approximately 90 percent are saccular (berry aneurysms). Saccular aneurysms are responsible for most of the morbidity and mortality caused by subarachnoid hemorrhage.11
Saccular aneurysms develop from defects in the muscular layer (tunica muscularis) of arteries. Alterations in the internal elastic membrane (lamina elastica interna) of cerebral arteries are thought to weaken vessel walls and render them less resistant to changes in intraluminal pressure.12 These changes most frequently develop at sites of vessel bifurcation, where blood flow is most turbulent and shear forces against the arterial wall are greatest13 (Figure 1).
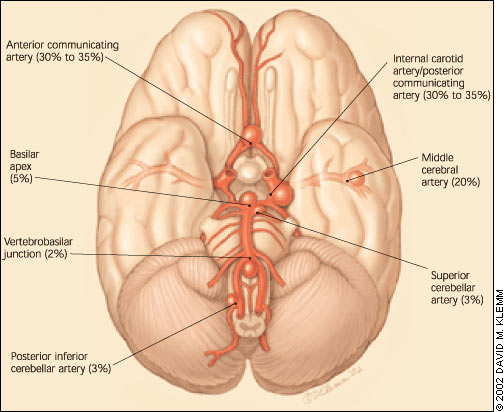
Saccular aneurysms most frequently form in first- and second-order arteries originating from the cerebral arterial circle (circle of Willis) at the base of the brain. Multiple aneurysms develop in 30 percent of affected patients.14
Fusiform aneurysms develop from ectatic, tortuous cerebral arteries, most often in the vertebrobasilar system, and can reach several centimeters in diameter. Patients with fusiform aneurysms characteristically present with symptoms of cranial-nerve or brainstem compression, but the symptoms are not commonly associated with subarachnoid hemorrhage.14
Dissecting aneurysms are the result of cystic medial necrosis or a traumatic tear of an artery. Like dissecting aneurysms elsewhere in the body (e.g., dissecting aortic aneurysms), they form as blood courses through a false lumen while the true lumen is collapsed upon itself.12
Clinical Presentation
Most intracranial aneurysms are asymptomatic and remain undetected until the time of rupture. Subarachnoid hemorrhage, a medical emergency, remains the most common initial clinical presentation. It was the first symptom in 58 percent of patients in one series.15 A history of the abrupt onset of a severe headache of atypical quality (“the worst headache of my life”) is typical of subarachnoid hemorrhage. Headache onset may or may not be associated with brief loss of consciousness, nausea and vomiting, focal neurologic deficits, or meningismus.
Despite the characteristic history, subarachnoid hemorrhage is frequently misdiagnosed. Nearly one half of patients present with milder symptoms caused by a “warning leak” before full rupture of the aneurysm.16
A case-record review of 111 patients referred to a tertiary care center for the management of unruptured aneurysm found that only 41 percent of the aneurysms produced symptoms (Table 2).17 In most of these patients, symptoms persisted beyond two weeks and were more likely to occur in patients with larger aneurysms located in the posterior circulation.
| Symptom | Number of affected patients | |
|---|---|---|
| Acute | ||
| Severe headache | 7 | |
| Transient ischemia | 7 | |
| Seizures | 3 | |
| Oculomotor nerve palsy or vision loss | 2 | |
| Chronic | ||
| Noncatastrophic headache of different character than previous headaches | 18 | |
| Chronic loss of vision | 10 | |
| Unilateral optic neuropathy | 7 | |
| Motor weakness or cranial neuropathy not involving the eye | 4 | |
| Facial pain | 3 | |
Imaging
Current neuroimaging techniques for intracranial aneurysms include intra-arterial digital subtraction angiography, magnetic resonance angiography, computed tomographic angiography, and transcranial Doppler ultrasonography. Although intra-arterial digital subtraction angiography is the gold standard, it is an invasive test with a 1 percent risk of transient neurologic complications and a 0.5 percent risk of permanent neurologic complications.18 Caution must be exercised in using magnetic resonance imaging in a patient with a history of surgery, because surgical clips may pose a threat to the patient during the test. The sensitivity and specificity of imaging modalities are presented in Table 3.3
| Modality | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Magnetic resonance angiography | 69 to 100 | 75 to 100 |
| Computed tomographic angiography | 85 to 95 | Not reported |
| Transcranial Doppler ultrasonography | 50 to 91 | 87.5 |
Risk of Aneurysmal Rupture
Given the high mortality rate associated with ruptured intracranial aneurysms, determining the likelihood of rupture is critical to management decisions. Before 1998, several large studies2,19 found annual rupture rates of 1.4 to 1.9 percent for intracranial aneurysms; rates of rupture were higher when aneurysms were larger than 10 mm in diameter, symptomatic, or located in the posterior circulation.
Although larger aneurysms have consistently been shown to be at higher risk for rupture, smaller aneurysms should not be overlooked. In one series of 25 patients,20 intracranial aneurysms were less than 5 mm in diameter at the time of rupture in five patients and less than 9 mm in diameter in 11 patients. Hence, vigilant surveillance and neurosurgical referral are necessary in all patients with intracranial aneurysms.
The findings of the International Study of Unruptured Intracranial Aneurysms (ISUIA)21 were published in 1998. To date, the ISUIA is the largest retrospective evaluation of the risk of aneurysmal rupture. Examination of 2,621 patient records at 53 medical centers over 7.5 years yielded average annual rupture rates below those of previous estimates. Aneurysms less than 10 mm in diameter had an average annual rupture rate of 0.05 percent in patients with no history of subarachnoid hemorrhage; however, the rupture rate was 10 times higher for aneurysms of a similar size in patients with a history of subarachnoid hemorrhage. The annual rupture rate for larger aneurysms approached 1 percent.
The ISUIA data suggest that aneurysms with a diameter of 10 mm or more are at critical risk for rupture. The study findings also make a strong case for differentiating patients with aneurysms and a history of subarachnoid hemorrhage from those without such a history. Surveillance of patients from the ISUIA is ongoing and will help to define the risk of aneurysmal rupture over time.
Treatment of Intracranial Aneurysms
Patients with suspected or confirmed asymptomatic or symptomatic intracranial aneurysms should be referred to a neurosurgeon. The two options for invasive treatment are open craniotomy and endovascular treatment.
CRANIOTOMY AND OPEN REPAIR
Surgical intervention has a longer history of use and more outcome data. A recent meta-analysis22 reviewing the risks of surgical repair in 2,460 patients with 2,568 aneurysms found an overall mortality rate of 2.6 percent and a permanent morbidity rate of 10.9 percent.
The ISUIA study also had a prospective component that addressed the morbidity and mortality of surgical treatment. Outcomes were evaluated in 1,172 patients at 30 days and one year after aneurysmal repair (Table 4).21 Morbidity and mortality rates associated with surgical intervention exceeded the risk of rupture in patients with aneurysms less than 10 mm in diameter, even 7.5 years after diagnosis. The morbidity rates were higher than those previously cited, probably because the cognitive status of these patients was evaluated before and after surgery. Older age (especially age older than 64 years) was the only risk factor associated with higher morbidity and mortality with aneurysmal repair.21 [Evidence level B, observational study]
| Time after surgery | History of subarachnoid hemorrhage | Mortality rate (%) | Disability rate (%) |
|---|---|---|---|
| 30 days | Yes | 0 | 13.7 |
| No | 2.3 | 15.3 | |
| 1 year | Yes | 1 | 12.1 |
| No | 3.8 | 12 |
In a recent retrospective review23 of 366 patients who were treated at one institution, not only advanced age but also aneurysmal size and the experience of the surgeon were risk factors for decreased function after surgery. [Evidence level B, retrospective case review]
ENDOVASCULAR TREATMENT
Endovascular treatment for intracranial aneurysms has developed over the past decade. The Guglielmi detachable coil system is the only device that the U.S. Food and Drug Administration has labeled for endovascular treatment of ruptured and unruptured aneurysms. In this treatment, microwires are custom-fitted to occlude the vessel abnormality.
Some studies suggest that endovascular treatment of aneurysms may result in less procedural morbidity and mortality than conventional surgical techniques. In one series,8 however, only 54 percent of aneurysms were completely occluded by the procedure. It is not clear whether an aneurysm must be completely occluded to protect the patient against rupture,24 and data on long-term outcomes for endovascular treatment are lacking.
One ongoing study is comparing outcomes in patients with unruptured aneurysms who are treated with surgery versus coiling. Results from this study are not yet available.
Screening for Intracranial Aneurysms
Given the serious consequences of intracranial aneurysmal rupture and the emergence of new technologies that could aid in diagnosis and treatment before rupture, the prospect of screening is intriguing. In recent years, the literature has given some attention to the subject.
Screening of asymptomatic patients without risk factors does not appear to provide any benefits.25 Asymptomatic patients are defined by the absence of symptoms noted in Table 2.17 The relatively low incidence of intracranial aneurysms, their relatively low rate of rupture, and the potential complications of management make screening of asymptomatic patients inappropriate for the prevention of morbidity and mortality. Similarly, screening of patients with acquired risk factors, such as smoking or alcohol abuse, is not recommended.25 [Evidence level C, consensus opinion]
Screening of patients with a positive family history of ruptured intracranial aneurysm is controversial. Patients with one affected first-degree relative should be differentiated from those with more than one such relative. In one study,26 screening was performed in 626 patients with first-degree relatives who had an aneurysmal subarachnoid hemorrhage: 4 percent of these patients were found to have intracranial aneurysms, most of which were less than 5 mm in diameter. Of these patients, 18 underwent surgery, and 11 had decreased neurologic function after the procedure. The study found that although screening in this patient population increased estimated life expectancy by 2.5 years, it also resulted in an average of 19 years of neurologic morbidity.26 [Evidence level B, observational study] Based on data such as these, the Stroke Council of the American Heart Association does not recommend screening for aneurysms in patients who have only one first-degree relative with aneurysmal subarachnoid hemorrhage.21 [Evidence level C, consensus opinion]
The issue of screening in patients who have two or more family members with intracranial aneurysms is more complicated. Although several studies27,28 have advocated the use of screening in this patient population, the conclusions were based on higher aneurysmal rupture rates than the ISUIA study found. A more recent analysis29 indicated that screening does not reduce significant morbidity or mortality in these patients. Therefore, the decision on whether or not to screen for intracranial aneurysms in patients who have two or more first-degree relatives with documented subarachnoid hemorrhage is best decided on a case-by-case basis.21 [Evidence level C, consensus opinion] A definitive recommendation may emerge as more is learned about the risk of intracranial aneurysmal rupture and as the techniques for diagnosing and managing intracranial aneurysms improve.
Screening should also be considered in patients with rare conditions (e.g., autosomal dominant polycystic kidney disease) that are associated with an increased risk of aneurysms.7 However, the decision to screen these patients should be based on their overall health.
In patients with a history of aneurysmal subarachnoid hemorrhage, the annual rate of new aneurysm formation is between 1 and 2 percent, and the risk of aneurysmal rupture appears to be increased.30 Therefore, surveillance of these patients with magnetic resonance angiography or intra-arterial digital subtraction angiography may be justified.23 [Evidence level C, consensus opinion]