
Am Fam Physician. 2002;66(6):1015-1021
Hantavirus pulmonary syndrome (HPS) is a severe cardiopulmonary illness most often caused by the Sin Nombre virus, which is transmitted to humans by inhalation of aerosolized particles of rodent excreta or direct rodent contact. Although HPS is more common in the western United States, cases have been identified in 31 states. The illness begins as a nonspecific febrile prodrome, sharing many of its initial symptoms with other more common viral infections. Patients then quickly develop noncardiogenic pulmonary edema, respiratory failure, and shock. Characteristic laboratory findings include thrombocytopenia, a left-shifted leukocytosis, hemoconcentration, and presence of immunoblasts. The overall case fatality rate of HPS is approximately 40 percent. Diagnosis is confirmed by serologic identification of IgM and IgG antibodies to Sin Nombre virus. There is no specific therapy, but early recognition of HPS during the prodromal phase can expedite initiating cardiopulmonary support in an intensive care unit, which is associated with improved survival rates. Prevention of HPS involves avoiding contact with rodents and rodent habitats.
Hantavirus pulmonary syndrome (HPS) was first recognized during the spring of 1993 after a cluster of young, previously healthy individuals who lived in the southwestern United States acquired an acute, fatal cardiopulmonary illness. The early nonspecific symptoms of these patients uniformly resembled those of other more frequently seen benign viral infections, so the severity of these infections was not initially suspected. However, the febrile prodrome was followed by rapid, diffuse, noncardiogenic pulmonary edema and hemodynamic compromise resulting in an initial case fatality rate of 76 percent.1
The etiologic agent was subsequently determined to be a previously unrecognized hantavirus, Sin Nombre virus (SNV), and is now known to be the predominant cause of HPS in the United States.2 Currently, at least eight species of hantavirus are known to cause HPS in the Western Hemisphere, four of which are found in the United States. Each different virus is associated with a single rodent host species; the reservoir for SNV is the deer mouse (Peromyscus maniculatus), one of the most common small mammals in the United States.3 Additionally, the cotton rat and rice rat are the rodent vectors in the southeastern United States for the Black Creek Canal and Bayou viruses, respectively; the whitefooted mouse carries the New York virus in the northeast.
Before 1993, illness caused by hantaviral infection had never been documented in the Americas. In Europe and Asia, infection with hantaviruses was known to cause hemorrhagic fever with renal syndrome (HFRS), an entity clinically distinct from HPS. HFRS lacks the severe pulmonary involvement characteristic of HPS and instead exhibits coagulaopathies and renal manifestations not usually seen in HPS.
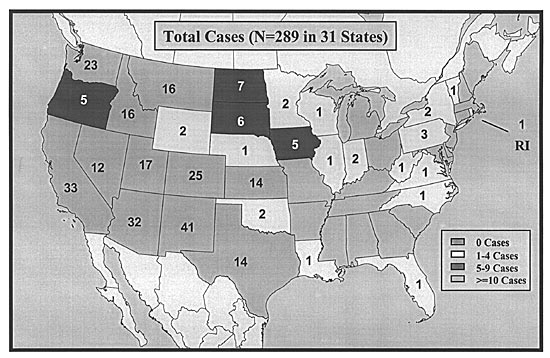
Since the initial outbreak in the United States, there have been more than 280 confirmed cases of HPS, representing approximately 30 cases per year. Contrary to common opinion, HPS is not limited to the southwestern United States and has been reported in 31 states (Figure 1),4 as well as in Canada and South America. This expanding geographic distribution is largely because of increased recognition of HPS. Fortunately, HPS remains a rare syndrome. However, because of its mild nonspecific prodrome and fulminant course, family physicians need to be aware of the epidemiology, clinical implications, clues to early diagnosis, and strategies for prevention of HPS.
Epidemiology
The Centers for Disease Control and Prevention (CDC) tracks the latest counts and descriptive statistics of HPS in the United States. CDC data indicate that the infection predominantly affects people in rural areas, is most common in the spring and summer, and carries an overall case fatality rate close to 40 percent. The age range of confirmed case patients is 10 to 75 years, and the mean age is 37 years.4 HPS is infrequently observed in children.
HPS is not a disease of any one racial group. Whites currently account for the majority of cases, about 77 percent. Native Americans account for about 20 percent, blacks 2 percent, and Asians 1 percent.4 When considering ethnicity, about 11 percent of HPS cases have been reported among Hispanics.
Although initially regarded as a new infection, an early case of retrospectively identified SNV infection dates back to July 1959.5 Additionally, reports from Native Americans living in the Southwest suggest similar episodes of illness have occurred for several decades.
VIRAL TRANSMISSION
SNV is transmitted to humans predominantly by inhalation of aerosolized viral particles from rodent excreta or saliva. Other potential mechanisms include direct contact with infected rodents and rodent bites, although these appear to be rare.
Person-to-person transmission of SNV has never been documented in the United States, and nosocomial infection has not occurred.6,7 However, investigation of an HPS outbreak in Argentina caused by a different hantavirus, the Andes virus, found epidemiologic evidence supporting person-to-person transmission involving health care workers.8
RISK FACTORS
Human illness most often results from exposure to infected rodent excreta. In an early case control study,9 peridomestic cleaning, agricultural activities, and an increased number of rodents in or around households were shown to be associated with HPS. Entering buildings that are rarely opened or only seasonally opened may also result in exposure to mice and their excretions, contributing to infection. Local increases in precipitation are associated with HPS, presumably by triggering an increase in the rodent population.10
Occupationally related HPS appears to be uncommon, and travel to areas where hantavirus illness has been reported is not a risk factor for contracting the disease. The possibility of exposure during recreational activities is small and can be further reduced by limiting contact with rodents.
Clinical Manifestations
It is helpful to think of HPS as having two distinct phases. The first is a prodromal phase characterized by a relatively mild febrile illness, typically lasting three to five days. The second phase, referred to as the cardiopulmonary phase, is manifested by severe, rapidly progressive respiratory failure. Invariably, the transition from mild illness to life-threatening cardiopulmonary compromise is abrupt and dramatic.
PRODROMAL PHASE
Patients in the prodromal phase present with fever and myalgia, making prodromal HPS difficult to distinguish from other more common viral infections. Other early symptoms include gastrointestinal disturbances, headache, chills, and malaise.4 Physical findings on initial presentation include fever, tachypnea, and tachycardia, but the remainder of the examination is usually normal.
CARDIOPULMONARY PHASE
The cardiopulmonary phase is heralded by the acute onset of pulmonary edema. Increased pulmonary capillary permeability is central to this process and may be caused by endothelial damage in the lung microvasculature.11 The patient becomes increasingly tachypneic, develops a cough productive of copious, nonpurulent secretions, and progressive hypoxia ensues. Progression of respiratory distress is so rapid that patients usually require mechanical ventilation within 24 hours.
In severe cases, significant myocardial depression also occurs, resulting in low cardiac output and hypotension. The HPS-related hypotension has a distinctive hemodynamic profile characterized by a low cardiac index and high peripheral vascular resistance, the opposite of which is seen in septic shock caused by other pathogens.11 Mortality is related to worsening cardiac depression, progressive respiratory failure, and subsequent acidosis culminating in fatal arrhythmias.
In patients who survive, the recovery occurs almost as quickly as the decompensation. After several days of supportive care, the cardiopulmonary processes quickly resolve, and most patients are discharged within one week of hospitalization. However, most survivors exhibit a modest degree of small airways obstruction even one year after recovery.
ATYPICAL PRESENTATIONS
Early studies indicated that essentially all patients infected with SNV went through the prodromal and cardiopulmonary phases. Recently, there have been confirmed cases of SNV infection in which patients had typical prodromal symptoms but never developed cardiopulmonary involvement.12 These reports suggest that SNV causes a wider spectrum of disease than previously thought.
Diagnosis
HPS should be considered when patients present with fever and myalgia, especially if they have any of the aforementioned risk factors for exposure to SNV, or have recently encountered areas likely to be contaminated with rodent excretions. Alack of such history, however, does not eliminate HPS from the differential diagnosis.
Rash, rhinorrhea, pharyngitis, and conjunctivitis are rare in HPS and thus, argue against SNV infection.13 It is important to note that rash and conjunctivitis are often seen in patients with HPS in South America. If HPS is being considered, a complete blood count (CBC) and chest radiograph should be obtained. Local health departments should be contacted for all suspected cases of HPS.
LABORATORY FINDINGS
Complete Blood Count
Progressive thrombocytopenia is one of the most consistent laboratory findings in HPS, occurring in virtually all patients, and it is frequently present during the prodromal phase. Thrombocytopenia has been shown to be highly discriminatory between patients with HPS and those with other febrile illnesses.14
Other important hematologic abnormalities include a left-shifted leukocytosis, presence of immunoblasts, and hemoconcentration. This tetrad (thrombocytopenia, left shift, circulating immunoblasts, and hemoconcentration) is seldom seen in other viral infections. If HPS is suspected and the initial CBC is normal, it should be repeated in eight to 12 hours.
Other Laboratory Findings
Modest impairments of the coagulation profile are seen, but overt disseminated intravascular coagulation is rare. Hypoalbuminemia and lactic acidosis occur late and indicate severe disease. Although it was not reported in the 1993 outbreak, renal impairment can be a component of HPS. Abnormalities of kidney function have been predominant in disease caused by Black Creek Canal and Bayou viruses.15
Serologic Diagnosis
The diagnosis is confirmed by serologic identification of IgM and IgG antibodies to SNV. Assays are available at most state public health laboratories and at the CDC.
RADIOLOGIC FINDINGS
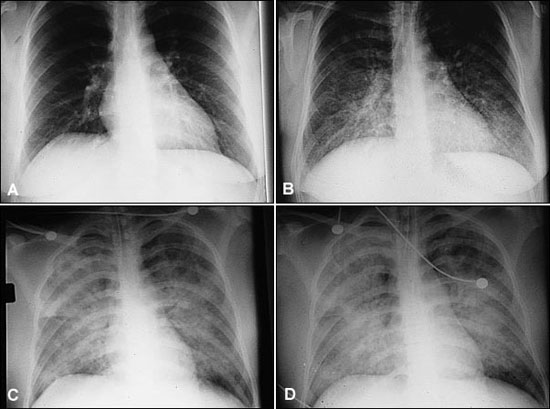
HPS has a characteristic radiologic evolution (Figure 2). Initial radiographs are most often normal, but approximately one third will exhibit mild interstitial changes. Heart size is normal, and there are no findings of pulmonary vasculature engorgement. Within 48 hours, basilar or central alveolar flooding occurs and pleural effusions are often seen. These findings differ from those seen in adult respiratory distress syndrome (ARDS), which has a more peripheral distribution and lacks the early prominent interstitial edema.16,17 A lobar infiltrate is more consistent with bacterial pneumonia.
DIFFERENTIAL DIAGNOSIS
A variety of processes can be confused with HPS. Infectious etiologies include pneumonia, sepsis with ARDS, and acute bacterial endocarditis. Several conditions, also more commonly seen in the southwest United States, have presentations similar to HPS, including septicemic plague, tularemia, histoplasmosis, and coccidioidomycosis. Noninfectious conditions, such as myocardial infarction with pulmonary edema and Goodpasture's syndrome, should also be considered.
Treatment
The probability of survival increases with early recognition and aggressive cardiopulmonary support.18 A high index of suspicion and an awareness of the early manifestations of hantavirus infection are essential to expedite rapid transfer to an intensive care unit. Currently, there is no specific therapy available to treat HPS, therefore the cornerstone of treatment remains supportive measures. Supplemental oxygen, mechanical ventilation when indicated, fluid management, and the appropriate use of pressors are crucial to patient care. Hemodynamic monitoring with a pulmonary artery (Swan-Ganz) catheter will facilitate fluid and pressor management and can aid in establishing a preliminary diagnosis by documenting the characteristic HPS hemodynamic profile. While awaiting serologic confirmation, broad-spectrum antibiotics should be initiated.
Two potential treatments are under investigation. One is the use of extracorporeal membrane oxygenation, which exhibited favorable results in a small study of patients with severe HPS.19 The second is the antiviral agent ribavirin (Virazole), which is currently being evaluated in a placebocontrolled clinical trial. An earlier open-label trial20 of ribavirin failed to demonstrate a significant improvement in outcome in patients with HPS. However, this study's design makes it difficult to assess the efficacy of ribavirin.
Prevention
Preventive strategies play a critical role in attempting to limit this lethal disease. The most effective way to decrease risk of HPS is to diminish human exposure to infected rodents; limiting peridomestic exposure is especially important. Measures to prevent HPS include eliminating rodents in and around households by keeping areas clean; rodent-proofing dwellings; controlling rodent populations through traps and rodenticides; properly cleaning areas of infestation; and avoiding rodents in outdoor settings.
Final Comment
Our current knowledge of HPS is in its infancy. We know relatively little about disease spectrum, pathogenesis, and potential therapeutic interventions. As the story continues to unfold, the CDC and state health departments remain excellent resources for new information.