A more recent article on premenstrual syndrome and premenstrual dysphoric disorder is available.
Am Fam Physician. 2002;66(7):1239-1249
From 2 to 10 percent of women of reproductive age have severe distress and dysfunction caused by premenstrual dysphoric disorder, a severe form of premenstrual syndrome. Current research implicates mechanisms of serotonin as relevant to etiology and treatment. Patients with mild to moderate symptoms of premenstrual syndrome may benefit from nonpharmacologic interventions such as education about the disorder, lifestyle changes, and nutritional adjustments. However, patients with premenstrual dysphoric disorder and those who fail to respond to more conservative measures may also require pharmacologic management, typically beginning with a selective serotonin reuptake inhibitor. This drug class seems to reduce emotional, cognitive-behavioral, and physical symptoms, and improve psychosocial functioning. Serotoninergic antidepressants such as fluoxetine, citalopram, sertraline, and clomipramine are effective when used intermittently during the luteal phase of the menstrual cycle. Treatment strategies specific to the luteal phase may reduce cost, long-term side effects, and risk of discontinuation syndrome. Patients who do not respond to a serotoninergic antidepressant may be treated with another selective serotonin reuptake inhibitor. Low-dose alprazolam, administered intermittently during the luteal phase, may be considered as a second-line treatment. A therapeutic trial with a gonadotropin-releasing hormone agonist or danazol may be considered when other treatments are ineffective. However, the risk of serious side effects and the cost of these medications limit their use to short periods.
Millions of women of reproductive age have recurrent emotional, cognitive, and physical symptoms related to their menstrual cycles. These symptoms often recur discretely during the luteal phase of the menstrual cycle and may significantly interfere with social, occupational, and sexual functioning.
Premenstrual dysphoric disorder (PMDD), a severe form of premenstrual syndrome (PMS), is diagnosed by the pattern of symptoms. According to a report by the Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists,1 up to 80 percent of women of reproductive age have physical changes with menstruation; 20 to 40 percent of them experience symptoms of PMS, while 2 to 10 percent report severe disruption of their daily activities. Menstruation-related physical discomfort, such as dysmenorrhea, may begin with menarche. Often this condition is superseded by PMS in late adolescence or the early 20s. These syndromes generally remain stable over time.
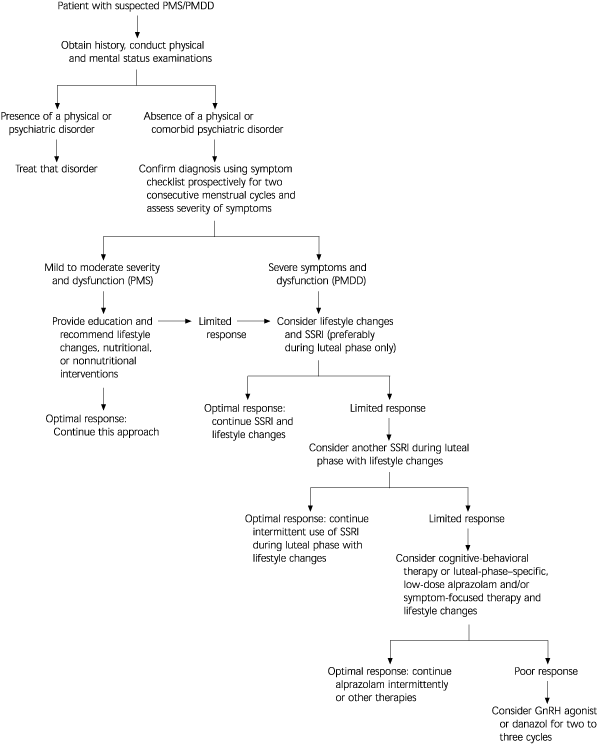
Diagnosis
In the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV), PMDD is classified as “depressive disorder not otherwise specified” and emphasizes emotional and cognitive-behavioral symptoms.2 At least five of the 11 specified symptoms must be present for a diagnosis of PMDD (Table 1).2 These symptoms should be limited to the luteal phase and should not represent amplification of preexisting depression, anxiety, or personality disorder. In addition, they must be confirmed prospectively by daily rating for at least two consecutive menstrual cycles. A symptom-free period during the follicular phase of the menstrual cycle is essential in differentiating PMDD from preexisting anxiety and mood disorders.
Researchers have developed a reliable and valid self-reporting scale called the Daily Symptom Report (see patient information handout).3 The report consists of 17 common PMS symptoms, including 11 symptoms from the DSM-IV PMDD diagnostic criteria. Patients rate each symptom on a five-point scale, from zero (none) to 4 (severe). The scale provides guidance for scoring the severity of each symptom and may be used in the office setting by primary care physicians for diagnosis and assessment of PMDD.
Etiology
Currently, there is no consensus on the cause of PMDD. Biologic, psychologic, environmental and social factors all seem to play a part. Genetic factors are also pertinent: 70 percent of women whose mothers have been affected by PMS have PMS themselves, compared with 37 percent of women whose mothers have not been affected.4 There is a 93 percent concordance rate in monozygotic twins, compared with a rate of 44 percent in dizygotic twins.4 Genetic influences mediated phenotypically through neurotransmitters and neuroreceptors seem to play a significant role in the etiology.
Features of PMDD and depressive disorders—specifically atypical depression—overlap considerably. Symptoms of atypical depression (i.e., depressed mood, interpersonal rejection hypersensitivity, carbohydrate craving, and hypersomnia) are similar to those of PMDD. Thirty to 76 percent of women diagnosed with PMDD have a lifetime history of depression,5 compared with 15 percent of women of a similar age without PMDD. A family history of depression is common in women diagnosed with moderate to severe PMS.6 There is significant comorbidity between depression and PMDD. Despite this relationship, many patients with PMDD do not have depressive symptoms; therefore, PMDD should not be considered as simply a variant of depressive disorder.7
The effectiveness of selective serotonin reuptake inhibitors (SSRIs), administered only during the luteal phase of the menstrual cycle,8–14 highlights the difference between PMDD and depressive disorder. Acute treatment with SSRIs increases synaptic serotonin without the down-regulation of serotonin receptors needed for improvement in overt depression. This finding suggests that PMDD is possibly caused by altered sensitivity in the serotoninergic system in response to phasic fluctuations in female gonadal hormone. Other studies also favor the serotonin theory as a cause of PMDD. In particular, the efficacy of l-tryptophan,15 a precursor of serotonin, and of pyridoxine,16 which serves as a cofactor in the conversion of tryptophan into serotonin, also favors serotonin deficiency as a cause of PMDD. Carbohydrate craving, often a symptom of PMDD, is also mediated through serotonin deficiency.
Because PMDD only affects women of reproductive age, it is reasonable to assume that female gonadal hormones play a causative role, possibly mediated through alteration of serotoninergic activity in the brain. Estrogen and progesterone seem to modulate levels of monoamines, including serotonin. Eliminating the effect of ovarian gonadal hormones through the use of a gonadotropin-releasing hormone (GnRH) agonist relieves PMDD symptoms.17 Subsequent administration of estrogen and progesterone causes symptoms to return in women with PMS but not in those without PMS symptoms.18
Treatment
LIFESTYLE CHANGES
Lifestyle changes may be valuable in patients with mildly severe symptoms and benefit their overall health. Aerobic exercise and dietary changes often reduce premenstrual symptoms.19,20 Decreasing caffeine intake can abate anxiety and irritability, and reducing sodium decreases edema and bloating. Many patients prefer to try lifestyle changes and/or nutritional supplements as a first step in the treatment of PMDD.
NUTRITIONAL SUPPLEMENTS
Many of the nutritional supplements described in Table 24,15,16,19–22 have proven efficacy. A meta-analysis16 of nine randomized, placebo-controlled trials was conducted to ascertain the effectiveness of vitamin B6 in PMS management. The researchers concluded that vitamin B6, in dosages of up to 100 mg per day, is likely to benefit patients with premenstrual symptoms and premenstrual depression. In another study,21 research literature (from January 1967 to September 1999) was reviewed to evaluate the effectiveness of calcium carbonate in patients with PMS. The reviewers concluded that calcium supplementation in a dosage of 1,200 to 1,600 mg per day is a treatment option in women with PMS. Calcium supplementation (using Tums E-X) was found to reduce core premenstrual symptoms by 48 percent in 466 patients.22 Vitamin E, an antioxidant, seems to reduce the affective and physical symptoms of PMS.20 Tryptophan,15 a substrate for serotonin, may also benefit some patients.15
| Lifestyle changes |
| Regular, frequent, small balanced meals rich in complex carbohydrates and low in salt, fat, and caffeine19,20 |
| Regular exercise19,20 |
| Smoking cessation20 |
| Alcohol restriction20 |
| Regular sleep20 |
| Nutritional supplements |
| Vitamin B6, up to 100 mg per day16 |
| Vitamin E, up to 600 IU per day20 |
| Calcium carbonate, 1,200 to 1,600 mg per day21,22 |
| Magnesium, up to 500 mg per day20 |
| Tryptophan, up to 6 g per day15 |
| Nonpharmacologic treatments |
| Stress reduction and management20 |
| Anger management4 |
| Self-help support group20 |
| Individual and couples therapy20 |
| Cognitive-behavioral therapy23 |
| Patient education20 about the cause, diagnosis, and treatment of PMS/PMDD |
| Light therapy20 with 10,000 Lx cool-white fluorescent light |
NONPHARMACOLOGIC TREATMENTS
Almost invariably, psychosocial stressors should be addressed, either as a cause or a result of PMDD. Psychosocial stressors are known to alter brain neurochemistry and stress-related hormonal activity. Stress reduction, assertiveness training, and anger management can reduce symptoms and interpersonal conflicts. Women with negative views of themselves and the future caused or exacerbated by PMDD may benefit from cognitive-behavioral therapy.23 This kind of therapy can enhance self-esteem and interpersonal effectiveness, as well as reduce other symptoms.23 Educating patients and their families about the disorder can promote understanding of it and reduce conflict, stress, and symptoms.20
HERBAL THERAPIES
A recent study24 reviewed efficacy and safety data on herbal supplements marketed for women. The author concluded that two herbal products, evening primrose oil and chaste tree berry, have been effective in treating PMS (Table 3).24–26 Other researchers25 have arrived at variable conclusions about the efficacy of evening primrose oil. It is thought to provide the gamma-linolenic acid required for synthesis of prostaglandin E1,24 one of the anti-inflammatory prostaglandins. Chaste tree berry may reduce prolactin levels,24,25 thereby reducing symptoms of breast engorgement. These herbal therapies have not been approved by the U.S. Food and Drug Administration (FDA) for use in PMDD, and their safety in pregnancy and lactation has not been established. Moreover, manufacturing standards for herbal products are not uniform.
| Herbal product | Dosage | Use recommendation | Comments |
|---|---|---|---|
| Evening primrose oil24,25 | 500 mg per day to 1,000 mg three times per day | Days 17 through 28 of menstrual cycle | Most-studied of all herbs used in treatment of PMS |
| May provide a precursor for prostaglandin synthesis | |||
| Benefits breast tenderness | |||
| Safety data in pregnancy and lactation lacking | |||
| Not approved for this use by the FDA | |||
| Chaste tree berry24–26 | 30 to 40 mg per day | Days 17 through 28 of menstrual cycle | May benefit breast symptoms |
| Inhibits prolactin production | |||
| Safety data lacking | |||
| Not approved for this use by the FDA |
PHARMACOLOGIC INTERVENTIONS
Antidepressant and Anxiolytic Medications
The serotoninergic antidepressants are the first-line treatment of choice for severe PMDD (Table 4).8–14,27–37 Fluoxetine, in a dosage of 20 mg per day, has been shown to be superior to placebo, whether used only during the luteal phase12 or throughout the full menstrual cycle.27–29 In a review29 of seven controlled and four open-label clinical trials of fluoxetine, symptoms were significantly reduced in patients with PMDD.
| Agents | Dosage | Use recommendation | Comments |
|---|---|---|---|
| SSRIs | |||
| Citalopram13,35 | 10 to 30 mgper day | Full cycle or luteal phase only | Benefits physical, cognitive, and emotional symptoms |
| Administration during luteal phase | |||
| Luteal-phase use is superior to continuous treatment | |||
| Not approved by FDA for this use | |||
| Fluoxetine12,27,29,35 | 20 mg per day | Full cycle or luteal phase only | Significant reduction of all symptoms |
| Decreased libido or delayed orgasm is most common side effect in long-term, continuous use | |||
| Approved by FDA for this use | |||
| Paroxetine30,35 | 10 to 30 mgper day | Full cycle | Benefits all symptoms |
| Transient GI and sexual side effects | |||
| Superior to maprotiline | |||
| Not approved by FDA for this use | |||
| Sertraline8–10,14,31–33,35 | 50 to 150 mg per day | Full cycle or luteal phase only | Benefits all symptoms |
| Transient GI and sexual side effects | |||
| Approved by FDA for this use | |||
| Other serotoninergic antidepressants | |||
| Clomipramine11,34 | 25 to 75 mgper day | Full cycle or luteal phase only | Benefits all symptoms |
| Anticholinergic and sexual side effects | |||
| Not approved by FDA for this use | |||
| Anxiolytics | |||
| Alprazolam28,36,37 | 0.375 to 1.5 mg per day | Luteal phase | Interrupted use during the luteal phase can reduce the risk of drug dependence |
| Use only if SSRIs are ineffective | |||
| Not approved by FDA for this use | |||
In one placebo-controlled study,30 paroxetine in a dosage of 10 to 30 mg per day improved mood and physical symptoms in patients with PMDD. Paroxetine was more effective than the noradrenaline reuptake inhibitor maprotiline.30 Sertraline in a dosage of 50 to 150 mg per day was superior to placebo whether used during the full menstrual cycle31–33 or only during the luteal phase.8–10,14 Citalopram in a dosage of 10 to 30 mg per day was effective in one randomized, placebo-controlled trial.13 Interestingly, intermittent administration of citalopram during the luteal phase was found to be superior to continuous treatment. Clomipramine, a serotoninergic tricyclic antidepressant that affects the noradrenergic system, in a dosage of 25 to 75 mg per day used during the full cycle34 or intermittently during the luteal phase,11 significantly reduced the total symptom complex of PMDD.
In a recent meta-analysis35 of 15 randomized, placebo-controlled studies of the efficacy of SSRIs in PMDD, it was concluded that SSRIs are an effective and safe first-line therapy and that there is no significant difference in symptom reduction between continuous and intermittent dosing. Because fluoxetine, citalopram, clomipramine, and sertraline were effective if administered during the luteal phase only, these drugs may be used as first-line therapy and taken intermittently only during the luteal phase. Such an approach can reduce the risk of long-term side effects (e.g., weight gain), minimize discontinuation syndrome, and reduce the cost of care. SSRIs benefit the total symptom complex of PMDD, not only the mood-related symptoms. It should also be noted that fluoxetine and sertraline are the only two SSRIs with FDA approval for use in the treatment of PMDD.
Alprazolam, a high-potency benzodiazepine with mood-enhancing and anxiolytic effects, has been shown to be somewhat effective in patients with PMS.28,36,37 Because of the potential for drug dependence, alprazolam should be considered a second-line drug and used only if SSRIs fail to achieve an optimal response. Therapy should be limited to the luteal phase, and the agent should be given in low dosages—0.375 to 1.5 mg per day. The risk of drug dependence with alprazolam can be minimized by administering it only during the luteal phase of the menstrual cycle in patients without a history of substance abuse.
Hormonal Therapies
It has been shown that by inducing anovulation and amenorrhea, GnRH agonists, leuprolide, histrelin, and goserelin provide significant relief of symptoms in patients without comorbid depression.38–40 However, these medications can induce menopausal symptoms such as hot flushes, vaginal dryness, fatigue, irritability, cardiac problems, and osteopenia. In women with a history of PMDD, treatment of induced menopause with estrogen39 or estrogen plus progestational agents18 can induce recurrent symptoms of PMDD. This finding supports the theory of an etiologic role for female gonadal hormones in PMDD.
Danazol (Danocrine), a weak androgen prescribed for patients with endometriosis, fibrocystic breast disease, and hereditary angioneurotic edema, is sometimes used to treat PMDD. The typical dosage is 100 mg twice a day. Such treatment can reduce symptoms but may result in anovulation and masculinization, either of which may limit regular use.41 Because of the potential for serious side effects and significant costs, GnRH agonists and danazol should be tried as a last resort. These medications must be initiated during menstruation to prevent teratogenicity if there is an unintended pregnancy.
Although oral contraceptive pills (OCPs) suppress ovulation, they are not reported to be consistently effective in the treatment of PMDD (perhaps because the studies had variable samples). OCPs may not suffice if mood symptoms are prominent and, in some patients, these drugs may worsen dysphoria (a known side effect of some birth control pills) in many women without PMDD.
Efficacy studies of progesterone have shown limited benefits. One study42 found progesterone to be superior to placebo; however, another study43 reported efficacy equal to or less than that of placebo. Currently, ovarian gonadal hormones are thought to be of limited usefulness in the treatment of PMDD, and none of the drugs has FDA approval for this indication (Table 5).18,20,38–43
| Drug | Dosage | Use recommendation | Comments |
|---|---|---|---|
| Leuprolide depot38,40 | 3.75 mg IM per month | Up to six cycles | Pregnancy category X |
| Significant relief from symptoms but can induce menopausal syndrome | |||
| Leuprolide depot with ovarian hormone supplements18 | 3.75 mg IM per month with estrogen and progesterone | Can exceed six cycles | Less likely to induce menopause; PMDD symptoms may return, making this combination less effective |
| Goserelin with estrogen supplementation39 | 3.6 mg SC every 28 days with estrogen | Can exceed six cycles | Less likely to induce menopause; PMDD symptoms may return, making this combination less effective |
| Pregnancy category X | |||
| Use nonhormonal contraception during therapy and for 12 weeks after discontinuation of drug or until menses resume | |||
| Danazol41 | 100 mg twice a day | Up to six cycles | May cause masculinization from weak androgenic properties |
| Pregnancy category X | |||
| OCPs20 | OCPs with varying amounts of estrogen and progesterone, once a day | Full cycle | Variable response; may not benefit patients with significant mood symptoms; in some patients, may make mood symptoms worse |
| Progesterone42,43 | Vaginal suppositories, 200 to 400 mg per day | Not recommended for this use | Questionable efficacy |
Miscellaneous Pharmacologic Interventions
In a double-blind, placebo-controlled, crossover study,44 spironolactone in a dosage of 100 mg per day was more effective than placebo in reducing irritability, depression, somatic symptoms, feelings of swelling, breast tenderness, and craving for sweets. Bromocriptine in a dosage of up to 2.5 mg three times per day may be beneficial in patients with cyclic mastalgia,4,20 although in one study45 it was not found to be effective. Ibuprofen, in a dosage of up to 1,000 mg per day, can reduce breast pain, headaches, back pain, and other pain symptoms,20 but seems to have limited effect on mood symptoms (Table 6).4,20,44,45
| Agents | Dosage | Use recommendation | Comments |
|---|---|---|---|
| Diuretics | |||
| Spironolactone44 | 100 mg per day | Luteal phase | Aldosterone antagonist |
| Potassium-sparing diuretic | |||
| Could improve physical and psychologic symptoms | |||
| Dopamine agonist | |||
| Bromocriptine4,20,45 | Up to 2.5 mg three times per day | Days 10 through 26 of menstrual cycle | May relieve cyclic mastalgia; evaluate hepatic and renal functions before initiation |
| NSAIDs | |||
| Ibuprofen20 | 500 to 1,000 mg per day | Days 17 through 28 of menstrual cycle | Take with food May relieve mastalgia |
Other Medical Interventions
Historically, surgical and radiation oophorectomies have been used to treat severe PMS, but these modalities have no role in the current management of PMDD.
| Recommended treatment | Efficacy in PMS/PMDD | Efficacy rating* | Comments/evidence |
|---|---|---|---|
| Lifestyle changes19,20 | PMS or PMDD | G | Health benefits without risks |
| Vitamin B616 | PMS or PMDD | B | Dosage > 100 mg per day may cause peripheral neuropathy |
| Vitamin E20 | PMS or PMDD | E | Antioxidant without significant risk |
| Calcium carbonate21,22 | PMS or PMDD | B | Placebo-controlled study supports benefits in moderate to severe PMS |
| Tryptophan15 | PMS or PMDD | B | Supported by a placebo-controlled study |
| Cognitive-behavioral therapy23 | PMS | A | Benefits documented; not many studies |
| PMDD | B | — | |
| Herbal therapies24,25 | PMS or PMDD | E | Safety in pregnancy and lactation not documented; not FDA-approved |
| Selective serotonin reuptake inhibitors8–10,12–14,29–33,35 | Nonresponsive PMS or PMDD | A | Well-designed, randomized, placebo-controlled studies and metaanalyses |
| Clomipramine11,34 | PMDD | B | Anticholinergic side effects |
| Alprazolam28,36–37 | PMDD | B | Low-dose, luteal phase treatment; long-term use may cause tolerance |
| GnRH agonists or danazol18,38,39,41,42 | PMDD | C | Menopausal syndrome/masculinization/cost limit its use |
| Spironolactone, bromocriptine, or ibuprofen41,44,45 | PMS or PMDD | D | Symptom-focused efficacy; spironolactone efficacy supported by double-blind study |
| Oral contraceptives or progesterone42,43 | PMDD | E | Anecdotal efficacy or not consistently effective |
| Surgical or radiation oophorectomy | PMDD | F | Not recommended |