
Am Fam Physician. 2002;66(7):1269-1275
Patient information: See related handout on vulvar cancer, written by the authors of this article.
Vulvar cancer was reported in 3,200 women in 1998, resulting in 800 deaths. Recent evidence suggests that vulvar cancer comprises two separate diseases. The first type may develop from vulvar intraepithelial neoplasia caused by human papillomavirus infection and is increasing in prevalence among young women. The second type, which more often afflicts older women, may develop from vulvar non-neoplastic epithelial disorders as a result of chronic inflammation (the itch-scratch–lichen sclerosus hypothesis). Although vulvar cancer is relatively uncommon, early detection remains crucial given its significant impact on sexuality. Diagnosis is based on histology; therefore, any suspicious lesions of the vulva must be biopsied. Excisional or punch biopsy can be performed in the physician's office. Clinicians must closely monitor suspicious lesions because delayed biopsy and diagnosis are common. Once diagnosed, vulvar cancer is staged using the TNM classification system. Treatment is surgical resection, with the goal being complete removal of the tumor. There has been a recent trend toward more conservative surgery to decrease psychosexual complications.
In 1998, approximately 3,200 women in the United States developed cancer of the vulva, and 800 women died of the disease.1 Over the past decade, an increase in vulvar intraepithelial neoplasia (VIN) and VIN-related invasive vulvar cancer has been noted in women younger than 50 years.2 Overall, vulvar cancer is relatively uncommon, accounting for 3 to 5 percent of female genital-tract malignancies.
Approximately 90 percent of vulvar tumors are squamous cell carcinomas. When such tumors are found early, the prognosis is quite good. Despite its infrequency, vulvar cancer remains an important female disease, because of its significant impact on sexuality. Over the past decade, numerous advances have been made in the management of vulvar cancer, with a trend toward more conservative surgery and improved psychosexual outcomes. Early detection and biopsy of any abnormal vulvar lesions by primary care physicians are imperative to achieve diagnosis of vulvar cancer in the early stages and improve subsequent morbidity and mortality.
Epidemiology
It has been suggested that vulvar cancer exists as two separate diseases. The first type involves human papillomavirus (HPV) infection, which leads to VIN and predisposes the patient to vulvar cancer. The second type involves vulvar non-neoplastic epithelial disorders (VNED) and advanced age, leading to cellular atypia and cancer (Table 1).3
Vulvar cancer most frequently occurs in women 65 to 75 years of age.1 It can develop in younger patients, and a recent review4 noted that approximately 15 percent of all vulvar cancers occur in women younger than 40 years of age. One study5 noted that in women younger than 50 years of age, the incidence of vulvar cancer has increased from 2 to 21 percent over the past 20 years.
Most of the vulvar cancers appearing in young women arise in a field of warty or basaloid VIN. An estimated 80 percent of untreated women with warty VIN develop invasive disease.5 Thirty percent of patients with vulvar cancer present at 70 years or older, and the rate increases with age, reaching a peak of 20 per 100,000 women by 75 years of age.3 This rate equals lifetime risks of acquiring vulvar carcinoma and dying as a result of it of 0.3 and 0.1, respectively.6
No specific medical factors have been clearly identified as causative. Hypertension, diabetes mellitus, and obesity have been found to coexist in up to 25 percent of patients, although they are not considered independent risk factors.7 In the past, syphilis and other granulomatous diseases have been associated with vulvar cancer.7 However, this association is not likely, given the significant decline in the incidence of syphilis since 1992.
Over the past decade, the prevalence of VIN in young women has increased significantly.2 VIN is clearly a premalignant finding (Figure 1) and is associated with HPV infection, particularly subtypes 16 and 18.8 One study8 evaluated tissue samples from 48 patients with vulvar cancer. These patients ranged in age from 45 to 88 years. HPV DNA was detected by polymerase chain reaction in 48 percent of the specimens, of which 96 percent were from subtypes 16 and 18. HPV detection was not associated with age, but 71 percent were associated with coexisting severe (grade 3) VIN.
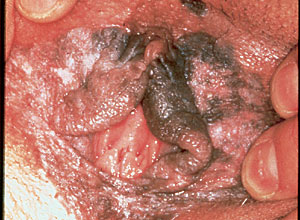
A retrospective review9 of women with vulvar cancer found a statistically significant correlation between patients younger than 45 years and HPV (relative risk [RR], 11.34), cigarette smoking (RR, 2.83), having more than two sexual partners (RR, 2.87), sexual initiation before age 19 years (RR, 2.43), and low economic status (RR, 1.77). In patients older than 45 years, there was a statistically significant correlation between VIN and vulvar cancer and VNED (RR, 23.6), residence in a rural area (RR, 2.17), low economic status (RR, 1.89), menopause before age 45 (RR, 1.84), poor hygiene (RR, 1.76), endocrine disorders (RR, 1.94), and low serum vitamin A levels (RR, 1.78).
Another study10 of women with vulvar cancer identified an odds ratio for vulvar cancer of 18.8 (confidence interval, 11.9 to 29.8) among current smokers who were HPV-16 seropositive, compared with women who had never smoked and were HPV-16 seronegative.
Lichen sclerosus, a type of VNED, is thought to be a predisposing factor in the development of HPV-negative vulvar cancer. According to the “itch-scratch–lichen sclerosus hypothesis,” lichen sclerosus, by causing a severe pruritus, sets up an itch-scratch cycle that over time causes the development of squamous cell hyperplasia.11 Further progression results in atypia formation, followed by VIN and eventual invasive squamous cell cancer.10 This hypothesis suggests that treatment of lichen sclerosus with topical steroids would prevent vulvar cancer in these patients, and some early research supports this suggestion.11 Aggressive evaluation and treatment of VNEDs could have a dramatic impact on the incidence of vulvar cancer in this subgroup of patients.
Clinical Features
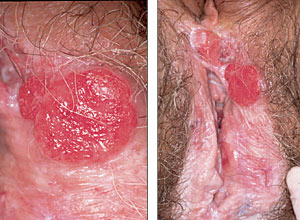
The most frequently reported symptom of vulvar cancer is a long history of pruritus. Less common presenting symptoms include vulvar bleeding, discharge, dysuria, and pain. The most common presenting sign of vulvar cancer is a vulvar lump or mass. Rarely, patients present with a large, fungating mass.
On physical examination, the vulvar lesion is usually raised and may be fleshy, ulcerated, leukoplakic, or warty in appearance7 (Figure 2). Most squamous cell carcinomas are unifocal and occur on the labia majora. Approximately 5 percent of cases are multifocal, and the labia minora, clitoris, or perineum may be involved as primary sites.
Screening
Any patient who reports or is found to have a vulvar lesion must be thoroughly evaluated to rule out malignancy. Both patients and physicians contribute to the delay in early and accurate diagnosis of vulvar cancer. According to one study,12 patients fail to seek treatment for symptomatic lesions or chronic pruritus for two to 16 months, while physicians often provide medical treatment of vulvar lesions for up to 12 months before obtaining a biopsy or considering referral.
Primary care physicians can identify women at risk and prevent serious, advanced disease by properly educating their peers and patients. Although there are no supporting data, expert opinion recommends routine annual visual inspection of the external genitalia, even if the patient is no longer receiving annual Papanicolaou smears.3 Teaching female patients about vulvar self-examination as part of their preventive health care regimen has also been advocated.3
Histologic Diagnosis
No gross features are diagnostic of vulvar cancer; diagnosis is based on biopsy alone. Therefore, biopsy must be performed on any suspicious lesions of the vulva, asymptomatic or symptomatic. Indications for biopsy include any grossly suspicious lesion such as a confluent, wartlike mass; persistent ulceration or itchy area; or change in the color, elevation, or surface of a lesion.12,13 Biopsy can be performed in the office under local anesthesia using excisional or punch biopsy.7 Patients with large or highly suspicious lesions can be referred to a gynecologist for biopsy, depending on the physician's level of comfort.
A punch biopsy down to the dermis is sufficient for pathologic evaluation. The biopsy should include surrounding skin with underlying dermis and connective tissue so that the pathologist can evaluate the depth of stromal invasion. Cutting down to the subcutaneous fat layer assures that the proper depth has been reached. This can be accomplished with a cervical punch biopsy instrument or a toothed pick-up and iris scissors. A small amount of local anesthetic with epinephrine will provide good analgesia and hemostasis. If additional hemostasis is needed, Monsel's solution or silver nitrate can be applied, although they may discolor the area. In rare cases, a single 3-0 or 4-0 absorbable suture may be necessary.
Delaying biopsy is the most common mistake made by clinicians. Although there are no data available to establish this standard, expert opinion recommends that any lesion increasing in size or with an unusual warty appearance be biopsied.7,12 Any typical condyloma that does not respond to therapy should be biopsied. The clinician must be diligent in observing any vulvar lesions and use biopsy aggressively on any lesion of concern to the clinician or patient.
The most common histologic type of vulvar cancer is squamous cell carcinoma (Table 2).7 The clinician should be aware of other histologic types that can present on the vulva, especially melanoma and verrucous carcinoma. Melanoma comprises 2 to 9 percent of malignant tumors of the vulva and 3 to 5 percent of skin melanomas.14 Verrucous carcinoma is significant because it is frequently misdiagnosed as a condyloma acuminatum. The latter tends to resist the usual means of eradication (e.g., podophyllin [Podofin], trichloroacetic acid, imiquimod [Aldara], surgery) and occurs most frequently in postmenopausal patients.
| Squamous cell carcinoma | |
| Melanoma | |
| Bartholin's gland carcinoma | |
| Adenocarcinoma | |
| Basal cell carcinoma | |
| Verrucous carcinoma | |
| Sarcomas | |
| Leiomyosarcoma | |
| Epithelioid sarcoma | |
| Rhabdomyosarcoma | |
| Lymphoma | |
| Endodermal sinus tumor | |
| Merkel's cell carcinoma | |
| Dermatofibrosarcoma protuberans | |
| Malignant schwannoma | |
Staging
Vulvar cancer is staged using the TNM classification system (Table 3).15 Staging reflects the characteristics of vulvar cancer growth, first by direct extension followed by lymphatic embolization to local lymph nodes, and finally by hematogenous spread to distant sites. Lymphatic spread, usually to the inguinal lymph nodes, can occur early in the disease process. From the inguinal nodes, the cancer spreads to the femoral nodes, followed by the pelvic nodes, and specifically to the external iliac chain.7
The incidence of lymph node metastasis is about 30 percent overall. The risk of nodal metastasis increases as the stage of disease, size of the lesion, and depth of invasion increase.7 Pelvic node metastases are uncommon, with an incidence of 2 to 12 percent, and are usually not found in the absence of clinically suspicious groin (inguinal and femoral) nodes.7 Twenty percent of patients with groin node metastases have positive pelvic nodes.
Treatment
Surgical resection is the gold standard of treatment in patients with vulvar cancer. Surgery should completely remove the cancer and identify the extent of disease to determine the stage and the need for additional therapy. The extent of disease determines the amount of surgery needed. Initially, radical vulvectomy with bilateral dissection of the groin and pelvic nodes was recommended as the standard treatment for most patients. Presently, a more individualized and conservative approach to treatment is recommended.
Most surgeons perform a radical vulvectomy or a radical local excision to treat vulvar cancer. The aim is to remove the primary lesion with a 1-cm margin and to remove the involved lymph nodes. The difference between a radical vulvectomy and a radical local excision is the bridge of skin between the lesion and the groin nodes. Metastases to the skin bridge were believed to be rare without clinically suspicious inguinal nodes; when radical vulvectomy was compared with radical excision, there was no difference in local recurrence.7
Groin node dissection is associated with wound infection and breakdown, and chronic leg edema. Research has found that stromal invasion of 1 mm or less is not associated with groin node metastases, but any patient who develops recurrent disease in an unresected groin has a high risk of mortality.7 The Gynecology Oncology Group recommends that any patient with more than 1 mm of dermal invasion have an inguinal–femoral lymphadenectomy; if more than two groin nodes are positive or there are clinically suspicious groin nodes (International Federation of Gynecology and Obstetrics [FIGO] stage N2), postoperative groin and pelvic radiation therapy is recommended.16
Recurrence in the groin appears to represent persistent disease, occurring early and near the treated site among patients treated conservatively.17 The risk of recurrent disease increases with the number of positive groin nodes. Patients with three or more positive nodes have a high incidence of local, regional, and systemic recurrence.7 Local recurrences are best treated with repeat surgical excision. Radiation therapy with surgery has been used to treat groin recurrence, while chemotherapy is used for systemic metastases. Chemotherapy has poor response rates and has been ineffective in the treatment of recurrent disease.7,12
Postoperative Complications
The family physician may occasionally encounter a patient who has undergone radical surgery for vulvar cancer. This physician is in a good position to provide support for recovery and surveillance of continued health after treatment. Table 47,12 lists the most common early and late postoperative complications. It is important that the atient be monitored for these complications and that treatment or referral be used in patients who develop complications. The clinician should be vigilant for local recurrence of cancer at the incision site, especially in the groin nodes, and watch for evidence of metastases. Signs of recurrence include skin breakdown, ulceration or mass in the area of previous cancer, or a new groin mass. Any new finding should be biopsied.
| Timing of complication | Complication |
|---|---|
| Early (up to six weeks fter surgery) | Anesthesia of anterior thigh (secondary o femoral nerve injury) |
| Deep venous thrombosis | |
| Groin wound infection and breakdown | |
| Hemorrhage | |
| Osteitis pubis | |
| Pulmonary embolism | |
| Seroma of the femoral triangle | |
| Urinary tract infection | |
| Wound necrosis | |
| Late (six weeks after surgery or later) | Chronic leg edema |
| Dyspareunia | |
| Femoral hernia | |
| Genital prolapse | |
| Recurrent leg lymphangitis | |
| Urinary stress incontinence |
Prognosis
The prognosis of patients with vulvar cancer is generally good when appropriate treatment is initiated in a timely fashion. The overall five-year survival is 70 percent and correlates with the stage of disease and lymph node status (Table 5).7,18 The number of positive groin nodes is the most important prognostic factor.7 Other factors that influence prognosis are tumor size and tumor ploidy (i.e., number of pairs of chromosomes). Patients with aneuploid tumors (i.e., an abnormal number of chromosomes) have a poorer five-year survival rate than patients with diploid tumors (23 versus 62 percent).19
| Stage/status | Five-year survival (%) | |
|---|---|---|
| Clinical FIGO stage | ||
| I | 98 | |
| II | 85 | |
| III | 74 | |
| IV | 31 | |
| Groin lymph-node status (all stages) | ||
| Positive | 52.4 | |
| Negative | 91.3 | |
| Positive pelvic nodes | 11 | |