
Am Fam Physician. 2002;66(12):2329-2335
The Recommended Adult Immunization Schedule1 for persons 19 years and older is the first harmonized schedule jointly approved by the Advisory Committee on Immunization Practices (ACIP), the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG). This schedule was developed in collaboration with members of AAFP, ACOG, the American College of Physicians/American Society of Internal Medicine (ACP-ASIM), ACIP, and the Centers for Disease Control and Prevention (CDC). Currently, the schedule is being reviewed by other adult health care organizations. The adult working group of the ACIP will annually review and update this schedule and provide recommendations to the ACIP and other organizations for their annual approval.
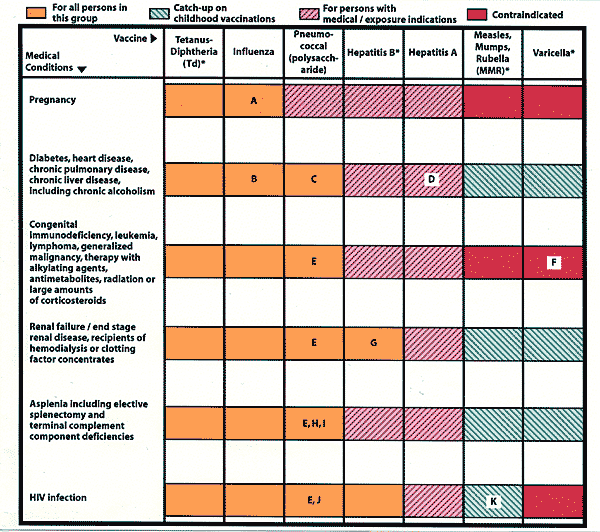
Similar to the Recommended Childhood Immunization Schedule,2 the Recommended Adult Immunization Schedule (Figure 1) provides age-based recommendations for routine vaccinations in a color-coded chart. In addition, it contains a color-coded chart that summarizes the recommendations and some contraindications of eight vaccines for adults with various medical conditions (Figure 2). The footnotes are summaries of the ACIP recommendations and also reflect any differences between health care organizations on use of these vaccines.

The success of the childhood immunization program is partly because of the annual publication of the Recommended Childhood Immunization Schedule that summarizes the current recommendations and that it can be posted in the office for quick reference. We suggest that physicians post the Recommended Adult Immunization Schedule in the office as a quick reference tool and that it be used as part of a larger office-based program to improve adult immunization rates.
One of the key immunizations on the schedule is the influenza vaccine. Taken together, influenza and pneumonia are the sixth leading cause of death nationally and the fifth leading cause in older adults. The fatality rate from influenza begins to rise in midlife and is highest in persons who have chronic medical conditions, such as chronic obstructive lung disease, cardiovascular disease, and diabetes mellitus, particularly if they are elderly. Many persons 50 to 64 years of age have a high-risk condition such as asthma, diabetes mellitus, or heart disease, but only a minority are vaccinated, despite recommendations that they should be.
Data from the 2000 National Health Interview Survey (www.cdc.gov/nchs/nhis.htm) show that only 32 percent of those 50 through 64 years of age who are at high risk for complications from influenza were vaccinated. Manual or computerized reminder systems based on high-risk conditions are more difficult to implement than those based on age. Many persons with a high-risk condition do not know that they have a high-risk condition, and high-risk vaccination strategies for other immunizations have had limited success. After considering these factors, the burden of influenza disease, and the cost-effectiveness of vaccination, in 1999 the AAFP became the first to lower the age for annual, routine influenza vaccination to age 50, a position that is now supported by other organizations.
Before vaccination, physicians should provide the patient with information about the benefits and the risks of adverse events of each vaccine to be administered. When administering any vaccine containing diphtheria, tetanus, pertussis, measles, mumps, rubella, poliovirus, varicella, hepatitis B, or Haemophilus influenzae type b antigens, the health care clinician is required to provide a copy of the relevant Vaccine Information Statement (VIS) to the patient before the vaccination. If there is not a VIS available for the vaccine being administered, the clinician should explain the risks of the disease, the protection afforded from the vaccination, the risk of vaccine adverse events, and what to do if a serious adverse event occurs.3 The following Web sites list every available VIS in multiple languages, which may be downloaded:www.cdc.gov/nip/publications/VIS/default.htm orwww.immunize.org/vis/index.htm. Physicians should report postvaccination adverse events to the Vaccine Adverse Event Reporting System, telephone: 800-822-7967.
Useful Web sites for current information include:www.immunizationed.org, a site developed by family physician educators, which will include free personal digital assistant software applications of the adult schedule;www.immunize.org;https://www.aafp.org/patient-care/clinical-recommendations/all/immunizations.html, which contains AAFP clinical policies on immunization;www.cdc.gov/nip; andwww.immunizationinfo.org.
The Recommended Adult Immunization Schedule complements the Recommended Childhood Immunization Schedule. Together, the two schedules provide a comprehensive summary of recommendations for prevention of vaccine-preventable diseases for Americans throughout their lifespan.