
Am Fam Physician. 2003;67(1):188-198
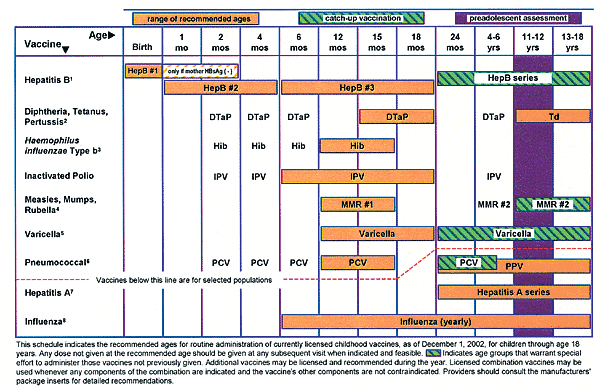
The 2003 Recommended Childhood and Adolescent Immunization Schedule (Figure 1) is similar to the 2002 schedule, except for four changes: a name change to reflect inclusion of adolescents, clarifications in the footnotes for hepatitis A and hepatitis B, encouragement for influenza vaccination of healthy children six to 23 months of age, and inclusion of a harmonized catch-up schedule for children who are behind in immunizations (Tables 1 and 2). The catch-up schedule offers specific guidance regarding the minimum time between doses as well as the number of doses for those who are behind schedule.
Among children zero to two years of age, influenza-related hospitalization rates range from about 186 to 1,038 per 100,000 for healthy children to 800 to 1,900 per 100,000 for those with high-risk conditions, depending on exact age.1–3 Izurieta and colleagues found rates of 144 to 187 per 100,000 children zero to 23 months of age.3,4 One study showed that healthy children six months to less than three years of age had rates of influenza-associated hospitalization as high or higher than rates among children three to 14 years of age with high-risk conditions.1,2 In one study,5 influenza was second only to respiratory syncytial virus in causing hospitalizations in persons with chronic underlying illness. Neuzil and colleagues1 found that for every 100 children, an annual average of six to 15 outpatient visits and three to nine courses of antibiotics are attributable to influenza. The illness attack rate is highest in children at 14 to 40 percent yearly, with attack rates typically higher than 30 percent in preschool-aged children.6–8
Influenza vaccine can cause local reactions such as soreness at the injection site. In young children not previously exposed to influenza vaccine, fever, malaise, and myalgia also can occur. Because inactivated influenza vaccines are not live, they cannot cause influenza. At the October 2002 Advisory Committee on Immunization Practices (ACIP) meeting, a study was presented from the Vaccine Safety Datalink that found that no serious reactions were associated with influenza vaccination among 251,600 children younger than 18 years, including 8,446 children six to 23 months of age, who received more than 438,000 doses of inactivated influenza vaccine.
Based on the hospitalization rates caused by influenza in young children, the high annual illness attack rate, and the safety of vaccination, the ACIP encourages vaccination of healthy children six through 23 months of age, beginning in the Fall of 2002.3 The Centers for Disease Control and Prevention's (CDC) Vaccine Information Statement on influenza has been updated to reflect this change (www.cdc.gov/nip/publications/VIS/default.htm). Before making a full recommendation to vaccinate all children six to 23 months of age annually (which is expected within the next two years), several issues need to be resolved, including parent and physician education, reimbursement, and efficient delivery mechanisms of influenza vaccine to young children.
Although vaccine shortages for tetanus, influenza, and varicella vaccines have resolved, shortages of conjugated pneumococcal vaccine continue. The ACIP recommends that children at highest risk (e.g., children with sickle cell disease) be vaccinated according to the normal schedule. During the shortage, the ACIP recommends that healthy infants and children younger than 24 months receive a decreased number of pneumococcal conjugate vaccine doses based on the age at which vaccination is begun and the estimated amount of vaccine available to the practice, as tabled atwww.cdc.gov/mmwr/preview/mmwrhtml/mm5050a4.htm.
| Minimum interval between doses | |||||||
|---|---|---|---|---|---|---|---|
| Dose one (minimum age) | Dose one to dose two | Dose two to dose three | Dose three to dose four | Dose four to dose five | |||
| DTaP (6 weeks) | 4 weeks | 4 weeks | 6 months | 6 months* | |||
| IPV (6 weeks) | 4 weeks | 4 weeks | 4 weeks† | ||||
| Hep B‡ (birth) | 4 weeks | 8 weeks (and 16 weeks after first dose) | |||||
| MMR (12 months) | 4 weeks§ | ||||||
| Varicella (12 months) | |||||||
| Hib‖ (6 weeks) | 4 weeks: if first dose givenat age <12 months | 4 weeks¶: if current age <12 months | 8 weeks (as final dose): this dose only necessary for children aged 12 months to 5 years who received three doses before age 12 months | ||||
| 8 weeks (as final dose): if first dose given at age 12 to 14 months | 8 weeks (as final dose)¶: if current age ≥12 months and second dose given at age <15 months | ||||||
| No further doses needed: if first dose given at age≥15 months | No further doses needed: if previous dose given at age ≥15 months | ||||||
| PCV# (6 weeks) | 4 weeks: if first dose given at age <12 months and current age <24 months | 4 weeks: if current age <12 months | 8 weeks (as final dose): this dose only necessary for children aged 12 months to five years who received three doses before age 12 months | ||||
| 8 weeks (as final dose): if first dose given at age ≥12 months or current age 24 to 59 months | 8 weeks (as final dose): if current age ≥12 months | ||||||
| No further doses needed: for healthy children if previous dose given at age ≥24 months | No further doses needed: for healthy children if first dose given at age ≥24 months | ||||||
Smallpox vaccination is not recommended for children in a pre-exposure situation because of the risk of adverse reactions. Studies from the 1960s reveal a death rate of one per 1 million primary vaccinations; rates of adverse reactions are highest among persons younger than five years. Adverse reactions include generalized vaccinia, inadvertent inoculation to other places on the body, eczema vaccinia that typically occurs among persons with a history of eczema, progressive vaccinia in persons with impaired T-cell function, postvaccine encephalitis (typically among infants and the elderly), transmission of vaccine virus to others, and death.
Useful Web sites for current information includewww.immunizationed.org, which is a site developed by family physician educators and has free Palm OS and CE applications of the childhood and adult immunization schedules,www.immunize.org,https://www.aafp.org/patient-care/clinical-recommendations/all/immunizations.html, which contains the American Academy of Family Physicians' clinical policies on immunization,www.cdc.gov/nip, andwww.immunizationinfo.org.
| Minimum interval between doses | ||
|---|---|---|
| Dose one to dose two | Dose two to dose three | Dose three to booster dose |
| Td: 4 weeks | Td: 6 months | Td*: |
| 6 months: if first dose given at age <12 months and current age <11 years | ||
| 5 years: if first dose given at age ≥12 months and third dose given at age <7 years and current age ≥11 years | ||
| 10 years: if third dose given at age ≥7 years | ||
| IPV†: 4 weeks | IPV†: 4 weeks | IPV† |
| Hep B: 4 weeks | Hep B: 8 weeks (and 16 weeks after first dose) | |
| MMR: 4 weeks | ||
| Varicella‡: 4 weeks | ||