
Am Fam Physician. 2003;67(2):315-322
Acute respiratory distress syndrome is a manifestation of acute injury to the lung, commonly resulting from sepsis, trauma, and severe pulmonary infections. Clinically, it is characterized by dyspnea, profound hypoxemia, decreased lung compliance, and diffuse bilateral infiltrates on chest radiography. Provision of supplemental oxygen, lung rest, and supportive care are the fundamentals of therapy. The management of acute respiratory distress syndrome frequently requires endotracheal intubation and mechanical ventilation. A low tidal volume and low plateau pressure ventilator strategy is recommended to avoid ventilator-induced injury. Timely correction of the inciting clinical condition is essential for preventing further injury. Various medications directed at key stages of the pathophysiology have not been as clinically efficacious as the preceding experimental trials indicated. Complications such as pneumothorax, effusions, and focal pneumonia should be identified and promptly treated. In refractory cases, advanced ventilator and novel techniques should be considered, preferably in the setting of clinical trials. During the past decade, mortality has declined from more than 50 percent to about 32 to 45 percent. Death usually results from multisystem organ failure rather than respiratory failure alone.
Acute respiratory distress syndrome (ARDS) refers to the syndrome of lung injury characterized by dyspnea, severe hypoxemia, decreased lung compliance, and diffuse bilateral pulmonary infiltrates. Family physicians can play an essential role in the early recognition of ARDS and contribute to the multispecialty team required to manage this life-threatening condition. This article reviews the current understanding of the pathophysiology, management, and prognosis of ARDS.
Originally referred to as traumatic wet lung, shock lung, or congestive atelectasis, ARDS was recognized in 1967 when the clinical, physiologic, radiographic, and pathologic abnormalities that were unique to a group of 12 patients were described, distinguishing them from other cases in a series of 272 patients treated for respiratory failure.1 A National Institutes of Health (NIH) panel initially estimated an incidence of 75 per 100,000 persons per year, but subsequent prospective studies give a range of 12.6 to 18 per 100,000 persons annually.2,3 Controversy still exists about the correct incidence because of differing criteria used to define ARDS. The prevalence of ARDS is reported to be between 15 and 18 percent of all ventilated patients.4 While a mortality rate greater than 50 percent is reported in the majority of clinical investigations performed between 1979 and 1994, more recent studies show a decline in mortality to be between 32 and 45 percent.2,5,6
Definitions of ARDS
Before 1992, the acronym ARDS represented the adult respiratory distress syndrome. The American-European Consensus Committee on ARDS standardized the definition7 in 1994 and renamed it acute rather than adult respiratory distress syndrome because it occurs at all ages. The term acute lung injury (ALI) was also introduced at that time. The committee recommended that ALI be defined as “a syndrome of inflammation and increased permeability that is associated with a constellation of clinical, radiologic, and physiologic abnormalities that cannot be explained by, but may coexist with, left atrial or pulmonary capillary hypertension.”7 Exclusion of left atrial hypertension as the primary cause of hypoxemia is critical to this definition, and measurement of pulmonary capillary wedge pressure may be necessary. The distinction between ALI and ARDS is the degree of hypoxemia,7 defined by the ratio of arterial oxygen tension to fractional inspired oxygen concentration (PaO2/Fio2), as shown in Table 1.8 ALI is defined by a ratio less than 300 mm Hg, and 200 mm Hg or less is required for ARDS.
Risk Factors
Multiple risk factors for the development of ARDS have been identified (Table 2).9 The sepsis syndrome appears to be the most common, but the overall risk increases with multiple factors.10 Blood transfusion is an independent risk factor.11 Advanced age and cigarette smoking are associated with an increased risk of developing ARDS, while alcohol consumption appears to have no influence.12 A review of the 1993 National Mortality Follow Back Study Database determined that the annual ARDS mortality is slowly declining, but that men and blacks have a higher mortality rate compared with women and other racial groups.13
Pathophysiology
In ARDS, the injured lung is believed to go through three phases: exudative, proliferative, and fibrotic, but the course of each phase and the overall disease progression is variable. In the exudative phase, damage to the alveolar epithelium and vascular endothelium produces leakage of water, protein, and inflammatory and red blood cells into the interstitium and alveolar lumen. These changes are induced by a complex interplay of proinflammatory and anti-inflammatory mediators.
Type I alveolar cells are irreversibly damaged and the denuded space is replaced by the deposition of proteins, fibrin, and cellular debris, producing hyaline membranes, while injury to the surfactant-producing type II cells contributes to alveolar collapse. In the proliferative phase, type II cells proliferate with some epithelial cell regeneration, fibroblastic reaction, and remodeling. In some patients, this progresses to an irreversible fibrotic phase involving collagen deposition in alveolar, vascular, and interstitial beds with development of microcysts.14
Clinical Presentation
About 50 percent of patients who develop ARDS do so within 24 hours of the inciting event. At 72 hours, 85 percent of patients have clinically apparent ARDS.3 Patients initially have tachypnea, dyspnea, and normal auscultatory findings in the chest. Some elderly patients may present with an unexplained altered mental status. Patients then become tachycardic with mild cyanosis and later develop coarse rales. They progress to respiratory distress with diffuse rhonchi and signs of consolidation, often requiring positive pressure ventilatory support. Even with significant hypoxemia, these clinical findings may not be obvious, so an arterial blood gas is warranted early in patients at risk. Initial oxygenation ratios and ventilatory parameters do not reliably predict the ultimate outcome in individual patients.
Imaging Studies
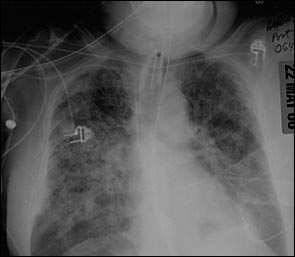
In patients with direct pulmonary insults, focal changes may be evident early on chest radiograph. In nondirect insults, the initial radiograph may be nonspecific or similar to congestive heart failure with mild effusions. Thereafter, interstitial pulmonary edema develops with diffuse infiltrates (Figure 1). As the disease progresses, the characteristic bilateral diffuse alveolar and reticular opacities become evident (Figure 2). Complications such as pneumothorax and pneumomediastinum may be subtle and difficult to identify, particularly on portable radiographs and in the face of diffuse pulmonary opacification. The patient's clinical course may not parallel the radiographic findings. With resolution of the disease, the radiographic appearance ultimately returns to normal.
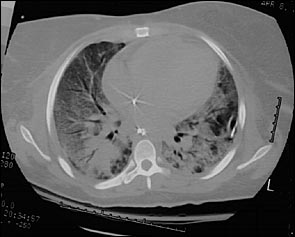
The predominant features of ARDS seen on a computed tomographic (CT) scan of the chest are diffuse consolidation with air bronchograms (Figure 3), bullae, pleural effusions, pneumomediastinum, and pneumothoraces. Later in the disease, lung cysts of varying number and size may be evident.15 Chest CT scan should be considered in patients whose respiratory status fails to improve despite correction of the inciting event. A CT scan can detect complications of ARDS and those related to catheter and tube placement such as pneumothorax, pneumomediastinum, focal pneumonia, catheter malposition, and pulmonary infarction.
Ventilator-Associated Lung Injury
An international consensus conference16 recognized that mechanical ventilation with high airway pressures may produce lung damage. Traditionally, barotrauma refers to air in extra-alveolar spaces such as pneumothorax and pneumomediastinum. More current concepts focus on the morphologic and functional changes at the epithelial and endothelial surfaces occurring before clinical extra-alveolar air.17 Volutrauma is the overdistension of injured and normal alveoli. The end-inspiratory volume is the key determinant of this overdistension and clinically correlates best with the inspiratory plateau pressure. Alveolar overdistension is believed to degrade surfactant, disrupt epithelial and endothelial cell barriers, and increase cytokine levels and inflammatory cells in the lung. Elevated ventilating pressures (barotrauma) also contribute to hydrostatic alveolar flooding.17 The severity of lung damage, as evident on CT scan of the chest, is associated with high inspiratory pressures and the duration of mechanical ventilation. The observed damage did not correlate with tidal volume or minute ventilation.18 Cyclic closure and reopening of injured alveoli may cause shearing of epithelial and endothelial cell layers.17
CONVENTIONAL MECHANICAL VENTILATION
Although patients with ARDS initially may be managed while breathing spontaneously with supplemental oxygen, hypoxemia is progressive and many patients require intubation and mechanical ventilation. Initial settings commonly used are the assist-control mode with provision of adequate positive end-expiratory pressure (PEEP). The use of high Fio2 concentration has been associated with pathologic changes in the lung such as edema, alveolar thickening, and fibrinous exudate.19 To avoid this toxicity, the Fio2 should be titrated toward 0.60 as long as oxygen saturation can be maintained at 90 percent or higher.
LUNG-PROTECTIVE STRATEGIES
PEEP increases the functional residual capacity and helps prevent alveolar collapse. It should be increased rapidly to keep Fio2 levels less than 0.60. The use of higher levels of PEEP (> 12 mm Hg) may be associated with a decrease in cardiac output, and monitoring of oxygen transport and cardiac parameters may be necessary.
Attempts to achieve a normal pH and partial pressure of carbon dioxide (PaCO2) by increasing the minute ventilation may predispose the patient to barotrauma and volutrauma. Permissive hypercapnia is a strategy of allowing gradual and modest increase in PaCO2 with reasonable acidosis. This is achieved by adjusting tidal volumes and respiratory rate to smaller minute volumes as a lung-protective strategy. A PaCO2 of 50 to 77 mm Hg and a pH of 7.20 to 7.30 seem well tolerated.20 The ARDS Network study21 reported a 22 percent reduction in mortality in patients who were managed with a low tidal volume (< 6 mL per kg) and low plateau pressure ventilator strategy. The use of higher respiratory rates and bicarbonate for acidosis had better results, compared with previous trials.21 It is recommended that limiting the airway pressure take priority over limiting the FIO2.22
In inverse-ratio ventilation, the inspiratory phase is longer than the expiratory phase. This is usually accomplished using pressure-control ventilation. This technique lowers the peak inspiratory and plateau pressure and increases the mean airway pressure. Inverse-ratio ventilation is uncomfortable and almost always requires patient sedation and paralysis. No clear advantage is evident over conventional techniques.20
ADVANCED STRATEGIES
Liquid ventilation is performed by filling the lung with a perfluorocarbon, a low surface tension liquid with a high affinity for oxygen and carbon dioxide. Suggested mechanisms of action are prevention of alveolar collapse in the filled lung, efficient removal of mucus and debris, and possible clearance of injury-producing cytokines. Liquid ventilation has shown an improvement in oxygenation and lung compliance when used in neonates.23 Clinical trials in adults with partial and full liquid ventilation are ongoing.
Nitric oxide is a potent endothelium-derived relaxing factor with reported antiplatelet and anti-inflammatory properties. Its use by inhalation in ARDS reduces the pulmonary arterial pressure and improves arterial oxygenation by improving blood flow to well-ventilated areas and decreasing intra-pulmonary shunting. Phase II and III trials with nitric oxide alone showed mild and transient improvement in oxygenation but failed to demonstrate improvement in mortality or reduction in ventilator days.24
Dependent consolidation is evident on CT scan in some patients with ARDS. The supine position favors hydrostatic collapse of posterior lung segments while prone positioning reverses this trend. No significant hemodynamic changes are associated with prone ventilation, but it requires adequate sedation. The risk of accidental dislodgment of essential monitoring devices is minimal, but dependent facial edema and development of pressure sores occur with prolonged therapy.25
Extracorporeal membrane oxygenation oxygenates blood through an artificial lung using a venovenous or arteriovenous circuit while resting the lung. In prospective studies, the use of extracorporeal membrane oxygenation alone has shown no advantage,26 but it may have a role in combination with other modes in patients who are refractory to other interventions. The major complication is potentially fatal bleeding. The combined therapy of controlled airway pressure, prone positioning, and inhaled nitric oxide may have better survival rates than the single modalities alone.27
Pharmacologic and Supportive Treatment
According to the American-European Consensus Committee,22 corticosteroids are not indicated in the early course of ARDS. A randomized study28 with relatively small numbers indicated that steroid use in the late or fibroproliferative phase might be beneficial. Initiating steroids before the development of end-stage fibrosis and aggressively ruling out ongoing infection appeared critical to success. In general, steroid use in ARDS is still controversial. Prophylactic antibiotics have no role in the management of ARDS. Studies with small groups of patients indicate that the use of beta agonists in patients with ARDS is safe, with a trend toward improved oxygenation and a decrease in peak and plateau ventilatory pressures.29
Pneumonia and other site-specific infections should be adequately treated with antibiotics based on appropriate culture and sensitivity. Sedation and paralytics may be required for patients who are managed with nonconventional modes of ventilation. However, frequent assessment of their depth and continued need is required to prevent prolonged paralysis. Optimizing fluid balance is prudent as long as systemic perfusion is objectively assessed and maintained, because the persistence of a positive fluid balance is associated with a poor prognosis.9 Routine stress ulcer and thromboembolic prophylaxis is recommended.
Maintenance of hemodynamic stability using fluid or inotropic agents may be required to ensure adequate perfusion. In various studies, the use of prostaglandins, antibodies, and receptor antagonists to various cytokines failed to reduce mortality in patients with ARDS. No advantage has been demonstrated with the use of N-acetylcysteine (Mucomyst) or aerosolized exogenous surfactant in multicenter trials.30,31 Drugs like nitroprusside (Nipride), hydralazine (Apresoline), prostaglandin E, and prostacyclin with vasodilatory properties similar to nitric oxide also have not been beneficial.9
Nutrition, preferably administered by the enteral route, is recommended. The inclusion of eicosapentaenoic acid from fish oil showed an improvement in ventilation requirements and length of stay for ARDS patients in the intensive care unit.32 Monitoring catheters and judicious use of antibiotics decreases nosocomial and super infections and length of stay in the intensive care unit. Secondary infections may account for nonresolution of ARDS and poor survival in patients with ARDS.22
Invasive Procedures
Although wedge pressure is part of the ARDS definition, routine use of pulmonary artery catheters is not indicated. These are now used selectively to optimize fluid and oxygen transport and when the clinical picture and chest radiograph suggest congestive heart failure. Pneumothorax may occur in up to 15 to 41 percent of patients with severe ARDS; however, data do not support the pro-phylactic placement of chest tubes.33 Pleural effusions that significantly impact ventilation need drainage by thoracentesis or chest tube. Bronchoscopy may be required when airway plugs result in major lung collapse and for proper specimen collection. Tracheostomy has been recommended in patients requiring prolonged intubation. Reported benefits include improved pulmonary toilet, decreased dead space, patient comfort, and decreased incidence of subglottic tracheal stenosis. Controversies still exist regarding the necessity and timing of the procedure. In refractory ARDS from indirect insults to the lung, an aggressive search for the inciting source is imperative. Abscess drainage or debridement of necrotic tissue may be necessary.
Prognosis
Usually, survivors start to recover within two weeks of the onset of ARDS.34 The overall mortality rate in ARDS is now about 32 to 45 percent, compared with 53 to 68 percent in the 1980s.9 It is possible that ventilator-induced injury may have accounted for the previously higher mortality rate. The aggressive management of initiating factors, concurrent infections, and improved nutritional support may also play a role in the declining mortality rate. Populations associated with higher mortality rates are the elderly, immunosuppressed persons, and patients with chronic liver disease.3 Age younger than 55 and trauma etiology predict a more favorable outcome.35,36 A multi-center review37 in the United States determined that patients older than 70 years had twice the likelihood of dying compared with their younger counterparts. An elevated dead space fraction has also been shown to be an independent risk for death.38 In cases of ARDS, death is usually caused by progressive multisystem organ failure rather than respiratory deterioration.34 Most survivors can lead fairly normal lives. Follow-up pulmonary function studies improve from three to six months and are stable at one year. Mild to moderate obstruction, diffusion, and restrictive abnormalities may persist, and careful follow-up is required in such patients.39 Neuropsychologic testing may reveal significant deficits in patients who had more severe and protracted hypoxemia.40
During the past 30 years, there has been considerable progress in standardizing the evaluation and management of this disease worldwide (Table 3). A better understanding of the pathophysiology has produced management strategies that have translated into evidence-based improvement in outcome. Experimental studies9 are being conducted to evaluate the role of epithelial growth factors and beta-adrenergic agonists in reducing lung injury and hastening repair. Investigation into the role of gene mutations and gene polymorphisms has given insight into possible genetic susceptibility for the development and outcome of ARDS.41 As more strategies and drugs are developed, there is hope that control of this once fatal disease will be possible. For now, treating the inciting cause, avoiding ventilator-induced injury, managing fluids judiciously, and providing supportive care remain the cornerstones of management.
| Perform early clinical determination of respiratory difficulty. |
| Perform objective assessment with arterial blood gas and chest radiograph. |
| Provide supplemental oxygen, monitor saturation, and investigate risk factors for ARDS. |
| Determine the need for intubation and mechanical ventilation. |
| Use low tidal volume, low plateau pressure, lung-protective ventilator strategies. |
| Optimize fluid status, nutrition, and pulmonary toilet, and treat complications. |
| Consider transfer to tertiary centers for clinical trials and advanced techniques. |