
Am Fam Physician. 2003;67(9):1927-1934
Recent events have demonstrated that bioterrorists have the ability to disseminate biologic agents in the United States and cause widespread social panic. Family physicians would play a key role in the initial recognition of a potential bioterrorism attack. Familiarity with the infectious agents of highest priority can expedite diagnosis and initial management, and lead to a successful public health response to such an attack. High-priority infectious agents include anthrax, smallpox, plague, tularemia, botulism, and viral hemorrhagic fever. Anthrax and smallpox must be distinguished from such common infections as influenza and varicella. Anthrax treatment is stratified into postexposure prophylaxis and treatment of confirmed cutaneous, intestinal, or inhalation anthrax. Disease prevention by vaccination and isolation of affected persons is key in preventing widespread smallpox infection. Many resources are available to physicians when a bioterrorism attack is suspected, including local public health agencies and the Centers for Disease Control and Prevention.
Bioterrorism emerged as a major U.S. public health concern in the fall of 2001, when 22 persons acquired confirmed cases of anthrax caused by the intentional release of anthrax spores.1,2 These events highlighted the importance of family physician recognition of unusual disease presentations and familiarity with potential bioterrorism agents.
The Working Group on Civilian Biodefense listed the following biologic disease agents as highest priority: anthrax, smallpox, plague, tularemia, botulism, and viral hemorrhagic fevers.3 These agents are more likely to be used in a bioterrorism attack than others because of the ease of dissemination or transmission, their potential to cause widespread panic, and the resource-consuming government responses that are required to investigate and manage such a bioterrorism attack.
The public health response starts with the family physician, who must have a high suspicion for these agents, be familiar with special diagnostic and management considerations, and notify public health authorities as soon as suspicion arises. Perhaps even more crucial is the ability of individual physicians to calm concerned patients and educate them about when treatment is actually warranted.
Anthrax
A bioterrorism attack occurred in the United States in the fall of 2001 when spores of Bacillus anthracis were intentionally distributed through the U.S. Postal Service to media and political outlets. Investigation of this attack provided new epidemiologic information on characteristics of anthrax disease.
Anthrax occurs naturally as a result of contact with anthrax-infected animals, such as sheep and cattle. The disease has three forms: cutaneous, inhalation, and gastrointestinal. Cutaneous anthrax is more common in naturally occurring infections, and inhalation anthrax is the predominant form when anthrax is released via an aerosolized route.1,4 The gastrointestinal form of anthrax infection is uncommon.
Cutaneous anthrax infection begins one to 14 days after exposure of a skin cut or sore to anthrax spores. The lesion presents as a painless, pruritic papule on exposed skin areas (Figure 1, left). During the next one to two days, vesicles may develop at the site (Figure 1, center). The vesicles rupture, and a painless, ulcerated, black eschar develops (Figure 1, right).4
Diagnostic tests for cutaneous anthrax infection include blood culture, Gram stain, and culture of the vesicular fluid and the base of the ulcer. A dry culture swab should be used to obtain the vesicular fluid, and a moist swab should be used for the base of the ulcer. A moist swab also can be used for the edge of the eschar.2 [Evidence level C, expert opinion]
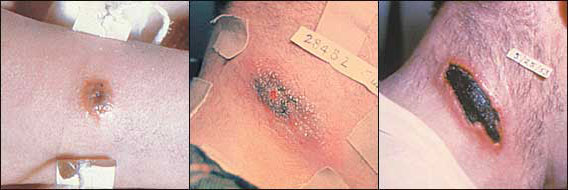
Physicians should consider punch biopsy if antimicrobial therapy has been initiated or if initial Gram stain and culture are negative for B. anthracis. If left untreated, this infection has a mortality rate of 20 percent4 (Table 1). Cutaneous anthrax is easily treated with antibiotic therapy5,6 (Table 2). Safety recommendations for the use of vaccines and antibiotics in women who are pregnant or breastfeeding are outlined in Table 3.6
| Disease (pathogen) | Incubation period | Mode of transmission | Mortality rate | Method of diagnosis |
|---|---|---|---|---|
| Anthrax (Bacillus anthracis)1,4 | 4 to 6 days | Cutaneous | Cutaneous: 20 percent, if untreated | Gram stain, blood or wound culture |
| Spores can remain dormant for up to 60 days. | Inhalation | |||
| Gastrointestinal (rare) | Inhaled: 45 percent | Rapid ELISA test | ||
| Smallpox (variola major and minor)9 | 12 to 14 days | Aerosolization | 30 percent | Clinical presentation of lesion |
| Direct contact | Electron microscopy of vesicular fluid | |||
| Fomite exposure | Virus cell culture | |||
| Plague (Yersinia pestis)16 | 2 to 8 days | Aerosolization | Pneumonic: almost 100 percent, if untreated | Clinical |
| Flea vector* | Gram stain of sputum, blood, or CSF | |||
| Wright's stain for bipolar (safety pin) staining | ||||
| Tularemia (Francisella tularensis)18 | 1 to 14 days | Aerosolization | < 2 percent | Sputum or blood culture |
| Rodent vector* | Sputum and blood for direct fluorescent antibody or immunohistochemical stains | |||
| Botulism (Clostridium botulinum)23 | 2 hours to 8 days | Aerosolization | Treated: < 5 percent | Clinical |
| Food contamination | Untreated: up to 60 percent | Serum bioassays | ||
| Viral hemorrhagic fevers (i.e., Lassa, Ebola, Marburg, Crimean-Congo)28 | 2 to 21 days | Aerosolization | 10 to 90 percent (depending on virus) | ELISA or IgM antibody detection |
| Rodent, mosquito, and tick vectors* | RT-PCR | |||
| Viral isolation |
Inhaled anthrax spores travel to alveoli, where macrophages may carry them to the mediastinal lymph nodes. Spores can remain dormant up to 60 days.4 The median incubation period in the bioterrorism-related inhalation anthrax outbreak in the fall of 2001 was four days (range: four to six days).1 During the initial phase of inhaled anthrax infection, patients may have nonspecific symptoms such as fever, sweats (often profound), fatigue, non-productive cough, dyspnea, and vomiting. Radiographs obtained during this initial phase are often abnormal and can help the physician distinguish anthrax infection from influenza. Radiographic findings can include mediastinal widening (Figure 2), paratracheal fullness, pleural effusions, hilar fullness, and infiltrates.
| Disease | Vaccine and dosage | Treatment (adults) | Treatment (children) | Prevention (adults) | |||
|---|---|---|---|---|---|---|---|
| Anthrax1,4 | Bioport vaccine, 0.5 mL SC at weeks 0, 2, and 4, and months 6, 12, and 18, then annual boosters | Ciprofloxacin (Cipro), 400 mg IV twice daily or Ciprofloxacin, 500 mg orally twice daily or Doxycycline (Vibramycin), 200 mg IV, then 100 mg IV twice daily for 60 days or Penicillin, 4 million units IV every four hours for 14 days | Ciprofloxacin, 10 to 15 mg per kg IV twice daily (maximum: 1 g per day) or Doxycycline (> 8 years), 2.5 mg per kg IV or orally twice daily for 60 days | Ciprofloxacin, 500 mg orally twice daily for 60 days; if unvaccinated, begin initial doses of vaccine orAmoxicillin, 500 mg orally three times daily Amoxicillin, 500 mg orally three times daily or Doxycycline, 100 mg orally twice daily for 60 days, plus vaccination | |||
| Smallpox9 | Wyeth calf lymph vaccinia, one dose by scarification | No current treatment other than supportive; cidofovir (Vistide) is effective in vitro; animal studies are ongoing | Same as adult treatment | Vaccinia immune globulin, 0.6 mL per kg IM within 3 days (most effective within 24 hours) of exposure | |||
| Plague16 | Vaccine is no longer available | Streptomycin, 30 mg per kg per day IM in two divided doses for 10 to 14 days or Gentamicin (Garamycin), 5 mg per kg IM or IV once daily for 10 to 14 days or Ciprofloxacin, 400 mg IV twice daily until clinically improved, then 750 mg orally twice daily for a total of 10 to 14 days or Doxycycline, 200 mg IV, then 100 mg IV twice daily until clinically improved, then 100 mg orally twice daily for a total of 10 to 14 days | Streptomycin, 15 mg per kg IM twice daily for 10 days (maximum: 2 g per day) or Gentamicin, 2.5 mg per kg IM or IV three times daily for 10 days or Doxycycline (< 45 kg), 2.2 mg per kg orally or IV twice daily for 10 days Doxycycline (> 45 kg), adult dosage | Doxycycline, 100 mg orally twice daily for seven days or duration of exposure or Ciprofloxacin, 500 mg orally twice daily for seven days or Tetracycline, 500 mg orally four times daily for seven days | |||
| Tularemia18 | Live attenuated vaccine (currently under review by the FDA), single 0.1-mL dose by scarification | Streptomycin, 7.5 to 10 mg per kg IM twice daily for 10 to 14 days or Gentamicin, 3 to 5 mg per kg per day IV for 10 to 14 days or Ciprofloxacin, 400 mg IV twice daily until clinically improved, then 500 mg orally twice daily for a total of 10 to 14 days or Ciprofloxacin, 750 mg orally twice daily for 10 to 14 days | Gentamicin, 2.5 mg per kg IM or IV three times daily for 10 days or Streptomycin, 15 mg per kg IM twice daily for 10 days (maximum: 2 g per day) or Ciprofloxacin, 15 mg per kg IV twice daily for 10 days (maximum: 1 g per day) | Doxycycline, 100 mg orally twice daily for 14 days or Tetracycline, 500 mg orally four times daily for 14 days or Ciprofloxacin, 500 mg orally twice daily for 14 days | |||
| Botulism23 | DOD pentavalent toxoid for serotypes A-E,* single 0.5-mL dose by deep SC at weeks 0, 2, and 12, then annual boosters | Botulism antitoxins available from CDC | Same as adult treatment | Not available | |||
| Viral hemorrhagic fevers28 | No licensed vaccine for any of the viral hemorrhagic fevers | Ribavirin (Virazole),* for Crimean-Congo, Lassa, Arenaviridae, and Bunyaviridae, 30 mg per kg IV (maximum: 2 g) initial dose, then 16 mg per kg (maximum: 1 g per dose) IV every six hours for 4 days, then 8 mg per kg (maximum: 500 mg per dose) IV every eight hours for six days | Same as adult treatment | Not available | |||
Computed tomographic scanning of the chest should be considered if inhalation anthrax is suspected because of its higher sensitivity for mediastinal lymphadenopathy.1,7 The white blood cell count is typically normal or slightly elevated with a left shift.1 Rapid diagnostic testing for influenza can be considered. Cultures of blood and pleural fluid should be obtained.
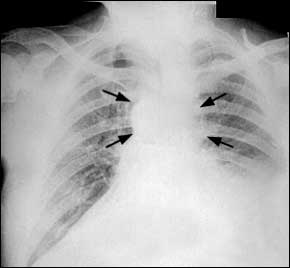
Notifying public health authorities of all confirmed cases of anthrax is mandatory. Laboratory resources from the Centers for Disease Control and Prevention (CDC) can provide confirmatory testing.2
The initial phase is followed by the abrupt development of clinical symptoms such as fever, diaphoresis, hemorrhagic meningitis, and shock. The mortality rate at this stage was 45.4 percent during the 2001 outbreak.1 Treatment options for inhalation anthrax are listed in Table 2. Persons with known exposure to anthrax spores should receive postexposure prophylaxis for at least 60 days with ciprofloxacin (Cipro) or doxycycline (Vibramycin) until antibiotic sensitivities are known.4 [Evidence level C, expert opinion]
A rapid diagnostic test (nasal swabbing) should be performed in patients strongly suspected of exposure to anthrax to confirm the diagnosis and determine antibiotic sensitivities.4 An anthrax vaccine was approved by the U.S. Food and Drug Administration (FDA) in 1970 and is used by the U.S. Department of Defense in military personnel.8
Smallpox
Smallpox is one of the highest-threat bioterrorism agents, with a fatality rate of 30 percent9 (Table 1). Smallpox infection has two forms: the more lethal variola major and the milder variola minor. The last reported case of smallpox occurred in 1977. Administration of the smallpox vaccine was halted in the United States in 1972, and persons who were vaccinated before then are no longer immune to infection. The Advisory Committee on Immunization Practices (ACIP) recommends voluntary vaccination of persons who may serve on smallpox public health response teams.10 [Evidence level C, expert opinion]
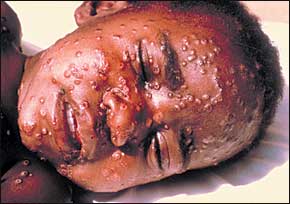
The deliberate introduction of the highly contagious smallpox virus via aerosolization or human carriers into a mobile population could cause a worldwide pandemic in just a few weeks. Following a 12- to 14-day incubation period (Table 1), smallpox infection presents abruptly with high fever, headache, backache, and malaise.9 Patients then develop a maculopapular rash on the face, forearms, and mucous membranes of the oropharynx (Figure 3). The rash then spreads to the trunk and legs. Within 48 hours, this centrifugal rash becomes vesicular. The variola form of smallpox can be differentiated from the more superficial and centripetal varicella (chickenpox) infection by the uniform appearance of the lesions and the involvement of the palms of the hands and soles of the feet11–13 (Table 4).11
At this time, no effective postexposure therapy is available (Table 2). Vaccine prophylaxis can prevent or lessen the severity of infection if administered within four days of exposure.14 Infection-control measures include early detection, isolation of infected persons, and public health surveillance of contacts.10
Plague
The plague, once called the “black death,” is caused by Yersinia pestis and has claimed millions of lives worldwide. Historians estimate that one third of the European population succumbed to the plague in the 14th century.15 Plague most commonly presents as the bubonic form but also can appear as septicemic or pneumonic forms.16 About 10 to 15 cases of the plague occur in the United States annually.17
The most common route of infection in humans involves transmission of the gram-negative bacillus following a plague-infected fleabite.16 The bubonic plague typically presents two to eight days after exposure, with sudden onset of fever, chills, weakness, and acutely swollen lymph nodes called buboes (usually in the groin, axilla, or cervical regions).16 Buboes can be extremely tender and are rarely fluctuant or suppurative. Symptoms without development of a bubo are termed primary septicemic plague.16
Person-to-person spread of bubonic or septicemic plague does not occur; however, pneumonic plague is highly contagious.16 Plague aerosolized as a weapon would result in the development of many cases of primary pneumonic plague. In 1970, a World Health Organization (WHO) committee reported that a 50-kg aerosol dispersal of Y. pestis in a population area of 5 million persons could result in 36,000 deaths and 80,000 to 100,000 hospitalizations.15
Pneumonic plague is by far the most deadly form, with almost 100 percent fatality if not treated with antibiotics (Table 1). Patients present with high fever, chills, malaise, and cough with bloody sputum (within 24 hours). Rapid treatment with antibiotics is essential in patients with pneumonic plague; delaying treatment until confirmatory testing is complete can decrease the chance of survival.16 Effective medications include streptomycin, gentamicin, ciprofloxacin, and doxycycline.5 No vaccine is currently available. Contacts should receive prophylaxis with antibiotics for seven days15 (Table 2).
Tularemia
Several countries have studied the possibilities of using F. tularensis as a biologic weapon.19 F. tularensis has many natural reservoirs, including rabbits, squirrels, muskrats, and cats; domestic rabbits are the primary source of infection for humans.20 Person-to-person transmission does not occur. In 1970, a WHO committee reported that a 50-kg aerosol dispersal of virulent F.tularensis in a population area of 5 million persons could result in 19,000 deaths and 250,000 hospitalizations.19 Bioterrorist dispersal could result in a large number of pleuropneumonitic cases. Without antibiotics, many cases would progress to respiratory failure and death.19
Tularemia can present in ulceroglandular, glandular, oculoglandular, oropharyngeal, typhoidal, or pneumonic forms.18 Pneumonic tularemia is the most severe presentation.21 The incubation period ranges from one to 14 days (Table 1). Symptom onset is rapid, with fever, headache, myalgia (particularly in the low back), sore throat, nausea, vomiting, diarrhea, and dry or slightly productive cough.12 The current mortality rate in the United States is less than 2 percent.18 First-line treatment options (Table 2) include aminoglycosides, macrolides, fluoroquinolones, and chloramphenicol.5,18 [References5 and18—Evidence level C, expert opinion] A live attenuated vaccine for tularemia is currently being reviewed by the FDA.22
Botulinum Toxin
Botulism is caused by Clostridium botulinum, which occurs naturally in soil. The toxin derived from this anaerobic, spore-forming bacterium has neuroparalytic effects.23 Most cases of botulism result from contaminated food being served undercooked; heat greater than 85°C (185°F) inactivates the toxin.24 The most likely bioterrorism dissemination scenarios include contamination of food and aerosolization.12,23
Botulism infection results from absorption of the neurotoxin through a mucosal surface, with intestinal absorption being much more common than lung and wound intoxication. Once the toxin is absorbed, it combines irreversibly with peripheral cholinergic synapses, resulting in acetylcholine release.24 Initial presentation includes gastrointestinal problems that rapidly progress to cranial nerve abnormalities (diplopia, dysphagia, dysarthria) and, particularly, bulbar deficits. A progressive, bilateral, descending motor neuron flaccid paralysis ensues, followed by respiratory failure and death.23 Mortality is less than 5 percent if the infection is treated but approaches 60 percent if it is untreated23 (Table 1).
Treatment consists of supportive critical care with assisted ventilation, prevention of secondary infection, and administration of an antitoxin.23 Botulism antitoxins are available from the CDC.23 The diagnosis of botulism is primarily clinical. It is important not to delay treatment for confirmatory testing, because the assays are not readily available18 (Table 2). A vaccine is available for laboratory workers and persons with increased risk of exposure; however, multiple doses are required to achieve immunity.23
Viral Hemorrhagic Fevers
Several geographically isolated viruses, including Lassa, Ebola, Marburg, and dengue, constitute the infectious agents for viral hemorrhagic fevers.25 Vectors include rodents, mosquitoes, and ticks.26 Aerosolization is a likely mode of terrorist dissemination.12 Incubation periods range from two to 21 days (Table 1), followed by abrupt onset of fever, myalgia, malaise, headache, vomiting, abdominal pain, and nonbloody diarrhea.27,28 A maculopapular rash on the trunk develops in most cases within five days. Later stage clinical manifestations include hepatic failure, renal failure, neurologic deficits, hemorrhagic diathesis, shock, and multi-organ dysfunction.27,28
| American Academy of Family Physicians (AAFP) www.aafp.org/btresponse.xml |
| American College of Emergency Physicians www.acep.org/1,4634,0.html |
| American College of Physicians (ACP)-American Society of Internal Medicine (ASIM) www.acponline.org/bioterro |
| Association for Professionals in Infection Control and Epidemiology, INC www.apic.org/bioterror |
| Centers for the Study of Bioterrorism & Emerging Infections (CSB&EI) www.bioterrorism.slu.edu |
| Centers for Disease Control and Prevention (CDC) www.cdc.gov |
| eMedicine www.emedicine.com |
| Johns Hopkins Center for Civilian Biodefense Strategies www.hopkins-biodefense.org |
| Medical NBC Online Information Server www.nbc-med.org/others/Default.html |
| National Academies www.nationalacademies.org |
| National Emergency Management Association www.nemaweb.org/sdp/Best_Practices/index.cfm |
| North Carolina Statewide Program for Infection Control and Epidemiology (SPICE) www.spice.unc.edu |
| Office of Justice Programs (OJ)/Office for Domestic Preparedness (ODP) www.ojp.usdoj.gov/odp |
| United States Army Medical Research Institute of Infectious Diseases (USAMRIID) www.usamriid.army.mil |
| Local health department phone number: |
| State health department phone number: |
Poor prognostic indicators include elevated liver function test results, bleeding, and neurologic involvement.26 Direct contact with bodily fluids and reuse of nonsterile needles cause the majority of cases.29 Isolation of the patient and notification of all suspected cases to the CDC or the local public health department are essential.
Treatment is primarily supportive. Ribavirin (Virazole) has shown limited success in the treatment of patients with viral hemorrhagic fevers such as Lassa, Crimean-Congo, some New World arenaviruses, and Bunya-viruses.25,28 The CDC has recommended giving ribavirin to patients with suspected viral hemorrhagic fever, pending final identification of the agent.29 [Evidence level C, expert opinion] Currently, there is no licensed vaccine for any of the viral hemorrhagic fevers.28
Table 5 lists Internet resources on bioterrorism recognition and management.