
Am Fam Physician. 2003;68(7):1311-1319
The primary treatment goals in patients with gastroesophageal reflux disease are relief of symptoms, prevention of symptom relapse, healing of erosive esophagitis, and prevention of complications of esophagitis. In patients with reflux esophagitis, treatment is directed at acid suppression through the use of lifestyle modifications (e.g., elevating the head of the bed, modifying the size and composition of meals) and pharmacologic agents (a histamine H2-receptor antagonist [H2RA] taken on demand or a proton pump inhibitor [PPI] taken 30 to 60 minutes before the first meal of the day). The preferred empiric approach is step-up therapy (treat initially with an H2RA for eight weeks; if symptoms do not improve, change to a PPI) or step-down therapy (treat initially with a PPI; then titrate to the lowest effective medication type and dosage). In patients with erosive esophagitis identified on endoscopy, a PPI is the initial treatment of choice. Diagnostic testing should be reserved for patients who exhibit warning signs (i.e., weight loss, dysphagia, gastrointestinal bleeding) and patients who are at risk for complications of esophagitis (i.e., esophageal stricture formation, Barrett's esophagus, adenocarcinoma). Antireflux surgery, including open and laparoscopic versions of Nissen fundoplication, is an alternative treatment in patients who have chronic reflux with recalcitrant symptoms. Newer endoscopic modalities, including the Stretta and endocinch procedures, are less invasive and have fewer complications than antireflux surgery, but response rates are lower.
Gastroesophageal reflux disease (GERD) is a common chronic, relapsing condition that is associated with a risk of significant morbidity and the possibility of mortality from complications. An estimated 44 percent of the U.S. adult population (61 million Americans) have heartburn, the hallmark of acid regurgitation, at least once a month.1 Approximately 14 percent of Americans have gastroesophageal symptoms weekly, and 7 percent have symptoms daily.1,2
Many patients self-diagnose and self-treat, and do not seek medical attention for their symptoms, while others have more severe disease, including erosive esophagitis.3 Patients who have GERD generally report decreased quality of life, reduced productivity, and decreased well-being. In many of these patients, reported quality of life is lower than in patients who have untreated angina pectoris or chronic heart failure.4 This article summarizes an evidence-based approach to the cost-effective management of patients with GERD.5
Diagnosis
A careful history is essential to establish the diagnosis of GERD. If a patient has classic symptoms of heartburn and acid regurgitation, the diagnosis can be made with high specificity, yet the sensitivity remains low.6,7 GERD can be missed in patients with heartburn, and some patients with Barrett's esophagus or adenocarcinoma of the esophagus do not complain of heartburn. Only 2 to 3 percent of acid reflux events reach the conscious level and are perceived by patients with GERD.8 Furthermore, many patients with GERD present with atypical symptoms6,7 (Table 1),9 although the presence of such symptoms is not required for clinical diagnosis.
| Asthma |
| Chest pain |
| Chronic cough |
| Dental enamel loss |
| Globus sensation |
| Initial onset of heartburn and regurgitation after 45 years of age |
| Recurrent laryngitis |
| Recurrent sore throat |
| Subglottic stenosis |
There is no gold standard for diagnosing GERD, although 24-hour pH monitoring (pH probe) is the accepted standard for establishing or excluding its presence. In patients with nonerosive reflux disease or symptomatic reflux esophagitis, 24-hour pH monitoring has a sensitivity and specificity of 70 to 96 percent, but false-positive or false-negative results are possible.10 While endoscopy lacks sensitivity for identifying pathologic reflux, it is the gold standard for assessing esophageal complications of GERD.11 Barium radiology is seldom useful for diagnosing GERD.12
In practice, the initial diagnosis of GERD is based on the history. Empiric acid suppression therapy for four to eight weeks should be tried in patients who have typical GERD symptoms without atypical manifestations and without warning signs or symptoms suggestive of complicated disease13,14 (Table 2).14 [Reference 13—evidence level A, meta-analysis of randomized controlled trials (RCTs)]
| Dysphagia |
| Early satiety |
| Gastrointestinal bleeding |
| Iron deficiency anemia |
| Odynophagia |
| Vomiting |
| Weight loss |
For the empiric trial, treatment may be initiated with a standard dosage of a histamine H2-receptor antagonist (H2RA) taken twice daily on demand or a standard dosage of a proton pump inhibitor (PPI) taken 30 to 60 minutes before the first meal of the day. The preferred empiric approach is step-up or step-down therapy. Step-up therapy begins with an eight-week trial of an H2RA and progresses to use of a PPI if symptoms of heartburn and regurgitation are not relieved. Step-down therapy starts with a PPI for eight weeks; treatment is then “downgraded” to the lowest effective dosage and type of medication that provide symptom relief.15
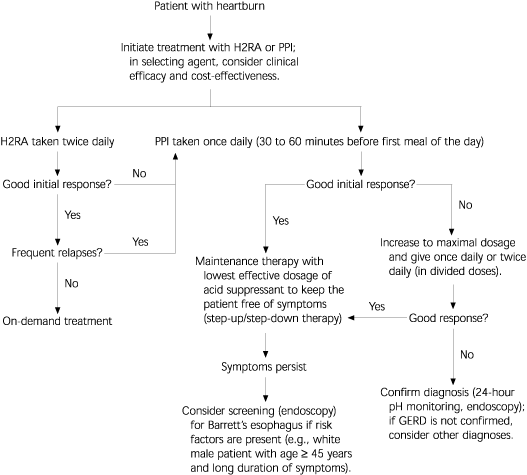
Drug selection should be based on the frequency or severity of symptoms at presentation, with a treatment goal of complete, cost-effective symptom relief13,14 (Figure 114 and Table 35). Diagnostic testing should be reserved for patients who present with warning signs and symptoms, have not responded to PPI therapy, or have disease duration of five to 10 years.
| Agent | Equivalent dosages | Dosage | Cost (generic)* | ||
|---|---|---|---|---|---|
| Histamine H2-receptor antagonists | |||||
| Cimetidine (Tagamet) | 400 mg twice daily | 400 to 800 mg twice daily | $109 (88) | ||
| Famotidine (Pepcid) | 20 mg twice daily | 20 to 40 mg twice daily | 121 (77 to 104) | ||
| Nizatidine (Axid) | 150 mg twice daily | 150 mg twice daily | 183 (165) | ||
| Ranitidine (Zantac) | 150 mg twice daily | 150 mg twice daily | 118 (91 to 95) | ||
| Proton pump inhibitors | |||||
| Esomeprazole (Nexium) | 40 mg per day | 20 to 40 mg per day | 132 | ||
| Lansoprazole (Prevacid) | 30 mg per day | 15 to 30 mg per day | 131 | ||
| Omeprazole (Prilosec) | 20 mg per day | 20 mg per day | 138 (112) | ||
| Pantoprazole (Protonix) | 40 mg per day | 40 mg per day | 104 | ||
| Rabeprazole (Aciphex) | 20 mg per day | 20 mg per day | 128 | ||
Treatment
LIFESTYLE MODIFICATIONS
Based on expert opinion, lifestyle modifications should be initiated and continued throughout the course of therapy in patients with a history that is typical of uncomplicated GERD (Table 4).14 Although there is little supporting evidence, it is considered reasonable to educate patients about various factors that may precipitate reflux.16
| Avoid large meals. |
| Avoid acidic foods (citrus- and tomato-based products), alcohol, caffeinated beverages, chocolate, onions, garlic, and peppermint. |
| Decrease dietary fat intake. |
| Avoid lying down within three to four hours after a meal. |
| Avoid medications that may potentiate GERD symptoms, including calcium channel blockers, beta agonists, alpha-adrenergic agonists, theophylline, nitrates, and some sedatives. |
| Elevate the head of the bed 10 to 20 cm (4 to 8 inches). |
| Avoid wearing clothing that is tight around the waist. |
| Lose weight. |
| Stop smoking. |
ANTACIDS
Over-the-counter acid suppressants and antacids are considered appropriate initial therapy for GERD. Almost one third of patients with heartburn-related symptoms use one of these agents at least twice weekly, for an annual expenditure of more than $1 billion.17,18 Antacids (e.g., Tums, Rolaids, Maalox) and combined antacid–alginic acid preparations have been shown to be more effective than placebo in relieving GERD symptoms, based on measures such as lower global symptom scores, less acid regurgitation, and fewer days and nights with heartburn.19,20
Sucralfate (Carafate), a prescription drug, increases the barrier to acid penetration in the esophagus. However, clinical studies have shown limited or no clinical efficacy for this agent in patients with GERD.14
HISTAMINE H2-RECEPTOR ANTAGONISTS
A number of RCTs have shown that H2RAs, given in standard dosages, are more effective than placebo for relieving heartburn in patients with GERD; within a few weeks of initiating treatment, up to 70 percent of patients reported symptomatic relief.13,14 No RCTs or systematic reviews have compared recurrence rates of esophagitis symptoms in patients treated with H2RAs or placebo.
A systematic review of 43 RCTs found faster healing rates in patients with erosive esophagitis who were treated with H2RAs compared with placebo.21 [Evidence level A, meta-analysis of RCTs] Higher dosages and more frequent dosing appear to increase the effectiveness of these agents in treating reflux symptoms and healing esophagitis.22 Disadvantages of using maximal dosages of H2RAs may include cost (possibly equal to or higher than the cost of PPI therapy) and poor compliance with the medication regimen.
The U.S. Food and Drug Administration has approved the use of cimetidine (Tagamet), famotidine (Pepcid), nizatidine (Axid), and ranitidine (Zantac) as over-the-counter preparations, with the dosage for each medication uniformly one half of the standard lowest prescription dosage. The four agents have similar clinical efficacy.
Some patients with GERD may be able to predict when they will have reflux symptoms. These patients may benefit from premedication with an over-the-counter H2RA. Alternatively, patients may elect to take the medication when symptoms occur (on-demand therapy). The over-the-counter H2RAs are believed to be more effective than antacids, alginic acid, and placebo.14
The efficacy of promotility agents is similar to that of H2RAs when given in standard dosages. Promotility agents can be used to augment therapy; however, they are seldom used because of their association with rare fatal cardiac arrhythmias.14
The course of incompletely treated GERD has not been examined in randomized trials. Little information is available on the degree of gastric acid suppression that is necessary to ensure adequate esophageal healing. Patients may develop tolerance to H2RAs, with some decrease in efficacy occurring after 30 days of therapy.
Dosages of H2RAs may need to be decreased in the elderly and in patients with renal insufficiency. In some case reports, these agents have been associated with rare cytopenias, gynecomastia, liver function test abnormalities, and hypersensitivity reactions. No RCTs have examined the safety of long-term H2RA therapy.
PROTON PUMP INHIBITORS
If a patient who was initially started on twice-daily H2RA therapy does not respond after two weeks, appropriate step-up therapy is to switch to once-daily PPI therapy (Figure 1).14 Evidence from several RCTs found that better control of reflux disease symptoms was achieved over a four-to eight-week period in patients treated with PPIs (83 percent) than in those given H2RAs (60 percent) or placebo (27 percent).14 Evidence also indicates that step-up therapy and step-down therapy are cost-effective and should be used.15,23 Furthermore, one study showed that a significantly greater number of patients treated with PPIs were in symptomatic remission at 12 months, compared with patients who were given H2RAs or placebo.24 [Evidence level B, uncontrolled trial]
In the treatment of erosive esophagitis, faster healing rates were achieved in patients who received PPI therapy for four to eight weeks (78 percent) than in patients who were given H2RAs (50 percent) or placebo (24 percent) for the same period.14 At one year, patients treated daily with a PPI were significantly less likely to relapse than those who received an H2RA.25
PPIs include lansoprazole (Prevacid), omeprazole (Prilosec), pantoprazole (Protonix), and rabeprazole (Aciphex). For these agents, no significant differences have been demonstrated in the symptomatic treatment of GERD or the healing of erosive esophagitis. Omeprazole recently became available in generic form, at only a slight reduction in cost compared with Prilosec. In the near future, an over-the-counter form of omeprazole should become available.
Esomeprazole (Nexium) is the S-isomer of omeprazole. Compared with omeprazole, esomeprazole is associated with higher rates of healing and symptom resolution in patients with GERD and reflux esophagitis.26 [Evidence level B, uncontrolled trial]
In patients with chronic or complicated GERD, the potential benefit of long-term PPI therapy generally outweighs the risk of adverse events. The most common side effects include headache and diarrhea. Rarely, cobalamin absorption is decreased, but a clinically significant decrease in serum vitamin B12 levels is unusual. The profound decrease in gastric acid secretion induced by PPIs leads to increased gastrin production from antral G cells. PPIs have not been linked to gastric cancer or carcinoid since their release more than 16 years ago.27
ANTIREFLUX SURGERY
Consideration of antireflux surgery must be individualized. Indications for surgery include failed medical management, patient preference for surgery despite successful medical management, complications of GERD, medical complications attributable to a large hiatal hernia, or atypical symptoms with reflux documented on 24-hour pH monitoring.
Potential surgical candidates should have reflux esophagitis documented by esophagogastroduodendoscopy and normal esophageal motility as evaluated by manometry. Patients being considered for surgery should have a defective antireflux barrier in the absence of poor gastric emptying. Potential candidates also should have at least a partial response to a previous trial of acid suppression therapy. The surgical referral should be made by a GERD subspecialist.
The basic tenets of surgery are the reduction of hiatal hernia, repair of diaphragmatic hiatus, strengthening of the gastroesophageal junction–posterior diaphragm attachment, and strengthening of the antireflux barrier through placement of a gastric wrap around the gastroesophageal junction (fundoplication). Surgery appears to be most effective for alleviating heartburn and regurgitation (beneficial in 75 to 90 percent of patients) and less effective for alleviating extraesophageal symptoms of cough, asthma, and laryngitis (beneficial in 50 to 75 percent of patients).28
While postsurgical complications are common, they are manageable in most patients. Approximately 10 percent of patients have solid food dysphagia; between 2 and 3 percent of these patients have permanent symptoms. From 7 to 10 percent of surgically treated patients have gas bloating; diarrhea, nausea, and early satiety occur more rarely. Although as many as 20 percent of patients have postsurgical complications, patient satisfaction is high when the symptoms of GERD are well controlled.29
Comparisons of antireflux surgery and antacid therapy in patients with erosive esophagitis have demonstrated marginal superiority for surgery as measured by heartburn relief, esophagitis healing, and improved quality of life. However, long-term follow-up studies have found that within three to five years of surgery, 52 percent of patients are taking antireflux medications again.30
NEWER ENDOSCOPIC TREATMENTS
The goals of radiofrequency heating of the gastroesophageal junction (Stretta procedure) and endoscopic gastroplasty (endocinch procedure) are to reduce medication use, improve quality of life, and decrease reflux symptoms in patients who have GERD, without the costs and risks associated with conventional antireflux surgery. Initial results for these treatments have been encouraging, with acid suppressant use decreased or eliminated in 50 to 75 percent of treated patients.31
To date, fewer than 10,000 patients have received any form of endoscopic antireflux treatment. Studies comparing postprocedure outcomes are currently in progress.
Follow-up
Further diagnostic testing should be performed in patients who have not responded to continuous therapy or who require such treatment, exhibit warning symptoms, or have risk factors for Barrett's esophagus.14 Although chronic reflux plays a major role in the development of Barrett's esophagus, it is not known if outcomes can be improved through surveillance coupled with surgical or medical treatment. In observational studies, progression to severe esophagitis has not occurred in patients with an initial normal endoscopy whose symptoms have remained unchanged during 10-year follow-up, thus arguing against repeat endoscopy during that time period.32