
Am Fam Physician. 2004;69(7):1691-1699
Retinal detachment often is a preventable cause of vision loss. There are three types of retinal detachments: exudative, tractional, and rhegmatogenous. The most common type is rhegmatogenous, which results from retinal breaks caused by vitreoretinal traction. Risk factors for retinal detachment include advancing age, previous cataract surgery, myopia, and trauma. Patients typically will present with symptoms such as light flashes, floaters, peripheral visual field loss, and blurred vision. Early intervention facilitates prevention of retinal detachment after formation of retinal breaks and improves visual outcomes of retinal detachment surgery. Patients with acute onset of flashes or floaters should be referred to an ophthalmologist.
Retinal detachment is relatively uncommon, affecting only one in 10,000 people per year, or approximately one in 300 patients in the course of a life-time.1 Retinal detachment often is repaired with little or no vision loss; therefore, it is a much less significant cause of irreversible blindness than other retinal diseases, such as diabetic retinopathy and macular degeneration.2 Retinal detachment should be considered in the differential diagnosis of vision loss, however, because it is more prevalent in defined subpopulations and may require urgent surgical repair.
Pathogenesis of Retinal Detachment
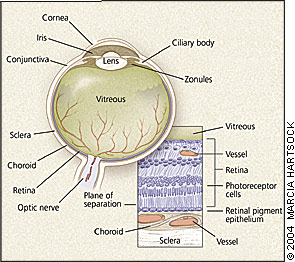
The retina is a neurosensory tissue that lines the interior posterior two thirds of the eye (Figure 1, left). The central retina, or macula, and the centermost macula, or fovea, exhibit structural and cellular specializations for fine central acuity.
Retinal detachment results when physiologic and anatomic mechanisms of retinal attachment are overcome and the retina separates from the underlying retinal pigment epithelium (Figure 1, lower right). Retinal detachments are classified into three pathogenetic types (Table 1). A patient may present with one or more of these types.
Exudative (or serous) retinal detachment results from the accumulation of serous and/or hemorrhagic fluid in the subretinal space because of hydrostatic factors (e.g., severe acute hypertension), or inflammation (e.g., sarcoid uveitis), or neoplastic effusions. Exudative retinal detachment generally resolves with successful treatment of the underlying disease, and visual recovery is often excellent.
The second type of detachment, tractional retinal detachment, occurs via centripetal mechanical forces on the retina, usually mediated by fibrotic tissue resulting from previous hemorrhage, injury, surgery, infection, or inflammation. Correction of tractional retinal detachment requires disengaging scar tissue from the retinal surface. In patients with tractional retinal detachment, vision outcomes are often poor.
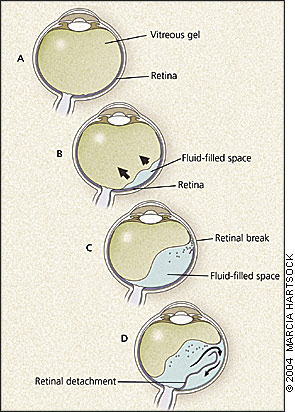
The third and most common type is rhegmatogenous retinal detachment. The key pathogenetic steps of rhegmatogenous retinal detachment are illustrated in Figure 2. The vitreous humor is a hydrated gel whose structure is maintained by a collagenous and mucopolysaccharide matrix (Figure 2a). As persons age, this macromolecular network begins to liquefy and collapse, the vitreous shrinks, and vitreo-retinal traction develops.3 Eventually, the vitreous partly separates from the retinal surface, which is known as posterior vitreous detachment (Figure 2b). Approximately one in four persons develops a posterior vitreous detachment between 61 and 70 years of age, and nearly two thirds have posterior vitreous detachment after 70 years of age.4 Posterior vitreous detachment is harmless by itself, though bothersome floaters (blood or retinal pigment epithelium cells) may develop and persist. However, in 10 to 15 percent of patients with symptomatic posterior vitreous detachment, a retinal flap tear or hole forms as the vitreous pulls away from the retina, especially in the periphery where the retina is thinner (Figure 2c).5
Retinal tears may occur without symptoms, but often photopsia (luminous rays or light flashes in vision) is noted. Photopsia results from mechanical stimulation of the retina by vitreoretinal traction. When the retina tears, blood and retinal pigment epithelium cells may enter the vitreous cavity and are perceived as floaters (Figure 2c). Patients with symptomatic retinal tears are at high risk of progression to retinal detachment.
Rhegmatogenous detachment occurs when liquid vitreous enters the subretinal space through a retinal break and creates a plane of dissection between the retina and retinal pigment epithelium (Figure 2d). Over time, the area of detachment increases as more fluid passes through the retinal break. Symptoms depend on the location and extent of the detachment. For example, a small detachment in the inferior retina results in a small superior visual field defect, while a large temporal detachment causes extensive nasal field loss. When the macula detaches, central acuity is lost. If untreated, nearly all rhegmatogenous retinal detachments progress to involve the macula.
Epidemiology of Retinal Detachment
The role of the vitreous in the pathogenesis of retinal breaks and detachments clarifies the risk factors for retinal detachment (Table 2). Detachment is more likely with advancing age because molecular breakdown and shrinkage of the vitreous humor increases over time.4,6 Previous cataract surgery commonly is associated with retinal detachment.7 The detachment occurs because following the surgical removal of the lens during cataract surgery, vitreous hyaluronic acid may pass into the anterior chamber and escape through the trabecular meshwork. Shrinkage and detachment of the vitreous are accelerated, and increase the risk of development to retinal tears. Retinal detachment occurs in approximately 1 percent of patients in the weeks to years following cataract surgery.8
| Type | Etiology | Management |
|---|---|---|
| Exudative (serous) | Inflammation (sarcoid uveitis), severe acute hypertension, neoplastic tumors | Treat underlying disease. |
| Tractional | Fibrosis* | Surgical excision of fibrosis |
| Rhegmatogenous | Retinal tears† | Surgery |
Myopia, or nearsightedness, is another leading risk factor for retinal detachment.9 In most patients with moderate or severe myopia, the axial (anteroposterior) length of the eye is greater, resulting in an egg-shaped globe. As a consequence, centripetal vitreoretinal traction increases and posterior vitreous detachment may occur at a younger age than in persons without myopia.4 Also, in myopic eyes, the retina is thinner and more prone to tear or hole formation at the time of posterior vitreous detachment. Therefore, persons with myopia are at risk of retinal detachment even in early adulthood; acute symptoms of flashes and/or floaters in these patients warrant a thorough fundus examination. Focal areas of peripheral retinal thinning and vitreoretinal adhesion occur less often in those without myopia, and are associated with retinal detachment.10
| Common | Less Common |
|---|---|
| Aging | Congenital eye diseases |
| Cataract surgery | Diabetic retinopathy |
| Focal retinal atrophy* | Family history of detachment |
| Hereditary vitreoretinopathy | |
| Myopia (axial) | Prematurity |
| Trauma | Uveitis |
Trauma precipitates retinal detachment for several reasons. At the moment of impact, rapid compression and decompression of the globe may generate sufficient vitreoretinal traction to produce retinal tears.11 Alternatively, retinal detachment may occur weeks, months, or even years after trauma because vitreous contraction is accelerated by inflammatory and blood-borne mediators that access the vitreous cavity at the time of injury.12 Despite these concerns, retinal detachment rarely follows head and periocular trauma,13,14 and fundus examination is warranted only if visual symptoms or external or radiographic evidence of ocular injury are present.
Less common associations with rhegmatogenous retinal detachment include a family history of detachment, a history of congenital eye diseases (such as glaucoma or cataract), hereditary vitreopathies with abnormal vitreous gel and high myopia (such as Stickler's syndrome), and previous retinopathy of prematurity (Table 2).
Evaluation of Patients with Suspected Retinal Detachment
A careful history helps to distinguish retinal detachment from other conditions with similar symptoms (Table 3). Floaters caused by acute posterior vitreous detachment, especially in the presence of a retinal tear, occur more abruptly and dramatically than do the floaters that people experience for much of their lifetime. Floaters are described by patients as fine dots, veils, cobwebs, clouds, or strings. Similar floaters occur with other causes of intraocular bleeding, such as proliferative diabetic retinopathy, trauma, and ocular inflammation (uveitis).
Light flashes may precede migraine headaches, but these typically occur bilaterally (though often in one area of the visual field). Photopsia that is induced by eye movements may indicate optic neuritis. Light flashes also may occur with postural hypotension and vasovagal reactions; these are bilateral and often accompanied by temporary dimming of vision and lightheadedness.
Visual field loss caused by retinal detachment begins suddenly, usually in the periphery, and progresses toward the central visual axis over hours to weeks; patients may describe this as a dim “shadow” or “curtain.” Field loss caused by stroke or other central nervous system processes is always bilateral, stable, and homonymous, due to crossing of nasal retinal projections at the optic chiasm. Even in patients with severe field loss caused by cerebral disease, the macula is spared and central vision persists. Visual loss from a transient ischemic attack may be unilateral, but is episodic, not persistent or progressive as in cases of retinal detachment, and may be accompanied by other neurologic symptoms. Fixed field defects of variable size occur in patients with retinal vascular occlusion; these patients often have hypertension or other atherogenic diseases, lack acute flashes, floaters, or other risk factors for retinal detachment, and may exhibit flame hemorrhage or arteriolar plaques by ophthalmoscopy.
| Symptom | Cause of symptom | Differential diagnosis |
|---|---|---|
| Photopsia | Vitreoretinal traction | PVD, optic neuritis, migraine, hypotension, transient ischemic attack |
| Floaters | Vitreous cells, blood | PVD, PDR, uveitis |
| Visual field loss | Peripheral detachment | Optic neuropathy, CVA, BRVO, BRAO |
| Blurred vision | Macular detachment |
In patients with optic nerve disorders, visual field loss is typically central or paracentral, pupillary reactions are diminished in the affected eye, and optic nerve head edema may be evident. Macular degeneration is the leading cause of painless sudden vision loss in older patients, but the scotoma is central and the peripheral field is intact. Common ocular signs and symptoms, such as pain, irritation, tearing, intermittent blurring, and conjunctival redness, are unrelated to retinal detachment.
Prompt referral and evaluation of patients who are suspected of having retinal tears are important because treatment of retinal tears is highly effective in preventing retinal detachment, and because progression of retinal detachment into the macula typically results in permanent visual loss. All patients with acute onset of flashes or floaters should be referred to an ophthalmologist (Table 4). If the patient has additional risk factors (e.g., myopia, previous cataract surgery, trauma, retinal detachment in the fellow eye, family history of detachment), suspicion of retinal detachment is heightened. If the patient exhibits acute visual field loss, whether subjective or demonstrable by confrontation, immediate referral is recommended. When the macula detaches, the potential to maintain normal vision is often lost. Thus, patients with extramacular detachment require more urgent management, even though they may present with excellent central visual acuity. These patients may benefit from direct referral to a retinal specialist to avoid the delay associated with additional examination by a general ophthalmologist or optometrist. In North America, most retinal detachment surgeries are performed by retinal specialists.
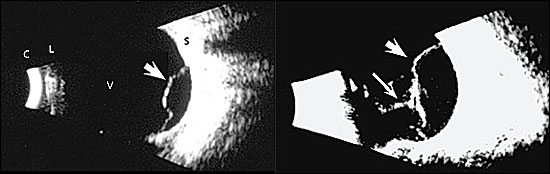
The direct (hand-held) ophthalmoscope is useful to detect an altered red reflex sometimes associated with retinal detachment. However, because its view is narrow, a normal examination with the direct ophthalmoscope cannot exclude a diagnosis of retinal detachment. Ophthalmologists use indirect examination techniques that greatly enhance visualization of the peripheral fundus. In cases where severe photophobia, periorbital edema, or opacity (e.g., cataract, intraocular blood) preclude ophthalmoscopy, ophthalmic ultrasonography is superior to magnetic resonance imaging and computed tomography for revealing occult retinal detachment (Figure 3).
Treatment of Retinal Detachment
Prevention is important in the treatment of retinal detachment. Protective eyewear is recommended for persons participating in contact sports, especially if they have moderate or severe myopia. Patients undergoing cataract surgery must be instructed about the importance of reporting symptoms of retinal tears and detachments.
The greatest opportunity for prevention exists in the hours to weeks following posterior vitreous detachment and retinal tear formation, because there is often a variable interval between retinal break and detachment.15 Only 1 to 2 percent of patients with a posterior vitreous detachment have a retinal break. If vitreous hemorrhage is present (often manifested as more marked floaters and blurring), this risk increases to 70 percent.16 Symptomatic retinal breaks are surrounded with laser or cryo burns to create a chorioretinal scar that prevents fluid access into the subretinal space. This treatment is over 95 percent effective in preventing progression of a retinal tear to retinal detachment17 (Figures 4a and 4b).
Surgical correction of retinal detachment aims to relieve vitreoretinal traction, and close retinal tears and holes. Scleral buckling techniques achieve reattachment in over 90 percent of cases18 (Figure 4c). An alternative means to relieve vitreoretinal traction is to remove the vitreous humor. This approach, called posterior vitrectomy, is successful in 75 to 90 percent of patients.19 Less invasive procedures, such as pneumatic retinopexy, allow repair of selected detachments in a clinic or office setting.20
If the central macula has not yet detached when the repair is achieved, visual acuity equal to preretinal detachment levels can be expected. However, if the central macula is detached at the time of repair, final visual recovery may vary from none to nearly complete, depending on the duration and degree of elevation of macular detachment and the patient's age. Therefore, surgical repair is indicated more urgently in patients with preserved central acuity, less urgently in patients whose macula detached in the previous hours to days, and routinely in those whose macula has been detached for several days or weeks21,22 (Table 4).Within months, photoreceptors in a detached retina suffer severe and irreversible damage caused by the separation from the underlying choroidal vascular supply (Figure 1, lower right), and repair yields less visual improvement.
Retinal detachment surgery fails in 5 to 10 percent of patients because of the growth of scar tissue on the retinal surface in the weeks following repair.23 Sources of fibrosis include blood cells, fibrin, inflammatory cells associated with postoperative healing, and retinal astrocytes and retinal pigment epithelium cells that enter the vitreous cavity when a retinal tear forms.24 Fibrotic tissue may exert sufficient inward traction to cause redetachment. This condition, known as proliferative vitreoretinopathy, is surgically corrected in 60 to 90 percent of patients, though visual acuity is often poor.25 Suppression of epiretinal fibrosis with antiproliferative agents is being intensively investigated, but remains elusive.26

| Presentation | Referral |
|---|---|
| Acute photopsias | Within one week |
| Acute floaters | Within one week, sooner if the floaters worsen |
| Visual field loss | Emergent, within one day, if possible |
| Any of above, chronic | Routine, within one to two months |
| Acute blurring | Urgent, within one to three days |
| Key clinical recommendation | Strength of recommendation | References |
|---|---|---|
| Therefore, surgical repair is indicated more urgently in patients with preserved central acuity, less urgently in patients whose macula detached in the previous hours to days, and routinely in those whose macula has been detached for several days or weeks. | B | 21,22 |