
Am Fam Physician. 2004;69(8):1915-1926
A more recent article on abnormal uterine bleeding in premenopausal women is available.
Abnormal uterine bleeding is a common presenting symptom in the family practice setting. In women of child-bearing age, a methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology. Dysfunctional uterine bleeding (anovulatory or ovulatory) is diagnosed by exclusion of these causes. In women of childbearing age who are at high risk for endometrial cancer, the initial evaluation includes endometrial biopsy; saline-infusion sonohysterography or diagnostic hysteroscopy is performed if initial studies are inconclusive or the bleeding continues. Women of childbearing age who are at low risk for endometrial cancer may be assessed initially by transvaginal ultrasonography. Post-menopausal women with abnormal uterine bleeding should be offered dilatation and curettage; if they are poor candidates for general anesthesia or decline dilatation and curettage, they may be offered transvaginal ultrasonography or saline-infusion sonohysterography with directed endometrial biopsy. Medical management of anovulatory dysfunctional uterine bleeding may include oral contraceptive pills or cyclic progestins. Menorrhagia is managed most effectively with nonsteroidal anti-inflammatory drugs or the levonorgestrel intrauterine contraceptive device. Surgical management may include hysterectomy or less invasive, uterus-sparing procedures.
Abnormal uterine bleeding is a common but complicated clinical presentation. One national study1 found that menstrual disorders were the reason for 19.1 percent of 20.1 million visits to physician offices for gynecologic conditions over a two-year period. Furthermore, a reported 25 percent of gynecologic surgeries involve abnormal uterine bleeding.2
Except for self-limited, physiologic withdrawal bleeding that occurs in some newborns, vaginal bleeding before menarche is abnormal.3 In women of child-bearing age, abnormal uterine bleeding includes any change in menstrual-period frequency or duration, or amount of flow, as well as bleeding between cycles.4 (Amenorrhea, or the cessation of menses for six months or more in nonmenopausal women, is beyond the scope of this article.) In postmenopausal women, abnormal uterine bleeding includes vaginal bleeding 12 months or more after the cessation of menses, or unpredictable bleeding in postmenopausal women who have been receiving hormone therapy for 12 months or more.5
This article presents a practical approach to determining the cause of abnormal uterine bleeding and briefly reviews medical and surgical management.
Etiology and Evaluation of Abnormal Uterine Bleeding
BEFORE MENARCHE
Malignancy, trauma, and sexual abuse or assault are potential causes of abnormal uterine bleeding before menarche. A pelvic examination (possibly under anesthesia) should be performed, because a reported 54 percent of cases involve focal lesions of the genital tract, and 21 percent of these lesions may be malignant.3
CHILDBEARING YEARS
The menstrual cycle has three phases. During the follicular phase, follicle-stimulating hormone levels increase, causing a dominant follicle to mature and produce estrogen in the granulosa cells. With estrogen elevation, menstrual flow ceases, the endometrium proliferates, and positive feedback is exerted on luteinizing hormone (LH), resulting in the ovulatory phase. During the luteal phase, progesterone elevation halts proliferation of the endometrium and promotes its differentiation; progesterone production by the corpus luteum diminishes, causing endometrial shedding, or menstruation. A menstrual cycle of fewer than 21 days or more than 35 days or a menstrual flow of fewer than two days or more than seven days is considered abnormal.6 (pp201–38)
| Pregnancy and pregnancy-related conditions | |
| Abruptio placentae | |
| Ectopic pregnancy | |
| Miscarriage | |
| Placenta previa | |
| Trophoblastic disease | |
| Medications and iatrogenic causes | |
| Anticoagulants7 | |
| Antipsychotics7 | |
| Corticosteroids7 | |
| Herbal and other supplements: ginseng, ginkgo, soy7 | |
| Hormone replacement | |
| Intrauterine devices | |
| Oral contraceptive pills, including progestin-only pills | |
| Selective serotonin reuptake inhibitors7 | |
| Tamoxifen (Nolvadex)7 | |
| Thyroid hormone replacement | |
| Systemic conditions | |
| Adrenal hyperplasia and Cushing's disease | |
| Blood dyscrasias, including leukemia and thrombocytopenia | |
| Coagulopathies | |
| Hepatic disease | |
| Hypothalamic suppression (from stress, weight loss, excessive exercise) | |
| Pituitary adenoma or hyperprolactinemia | |
| Polycystic ovary syndrome | |
| Renal disease | |
| Thyroid disease | |
| Genital tract pathology | |
| Infections: cervicitis, endometritis, myometritis, salpingitis | |
| Neoplastic entities | |
| Benign anatomic abnormalities: adenomyosis, leiomyomata, polyps of the cervix or endometrium | |
| Premalignant lesions: cervical dysplasia, endometrial hyperplasia | |
| Malignant lesions: cervical squamous cell carcinoma, endometrial adenocarcinoma, estrogen-producing ovarian tumors, testosterone-producing ovarian tumors, leiomyosarcoma | |
| Trauma: foreign body, abrasions, lacerations, sexual abuse or assault | |
| Dysfunctional uterine bleeding (diagnosis of exclusion) | |
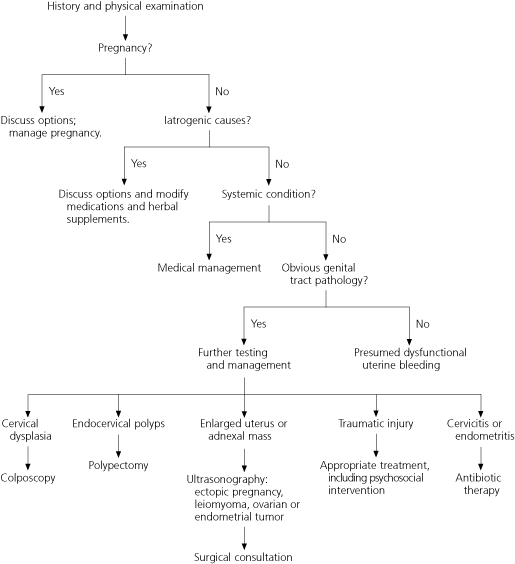
Pregnancy is the first consideration in women of childbearing age who present with abnormal uterine bleeding (Table 1).7,8 Potential causes of pregnancy-related bleeding include spontaneous pregnancy loss (miscarriage), ectopic pregnancy, placenta previa, abruptio placentae, and trophoblastic disease. Patients should be questioned about cycle patterns, contraception, and sexual activity. A bimanual pelvic examination (seeking uterine enlargement), a beta-subunit human chorionic gonadotropin test, and pelvic ultrasonography are useful in establishing or ruling out pregnancy and pregnancy-related disorders.
Next, iatrogenic causes of abnormal uterine bleeding should be explored. Bleeding may be induced by medications, including anticoagulants, selective serotonin reuptake inhibitors, antipsychotics, corticosteroids, hormonal medications, and tamoxifen (Nolvadex). Herbal substances, including ginseng, ginkgo, and soy supplements, may cause menstrual irregularities by altering estrogen levels or clotting parameters.9
| Diagnostic step | Pertinent signs, symptoms, and tests | Conditions |
|---|---|---|
| History | Pelvic pain | Miscarriage, ectopic pregnancy, PID, trauma, sexual abuse or assault |
| Nausea, weight gain, urinary frequency, fatigue | Pregnancy | |
| Weight gain, cold intolerance, constipation, fatigue | Hypothyroidism | |
| Weight loss, sweating, palpitations | Hyperthyroidism | |
| Easy bruising, tendency to bleed | Coagulopathy | |
| Jaundice, history of hepatitis | Liver disease | |
| Hirsutism, acne, acanthosis nigricans, obesity | Polycystic ovary syndrome | |
| Postcoital bleeding | Cervical dysplasia, endocervical polyps | |
| Galactorrhea, headache, visual-field disturbance | Pituitary adenoma | |
| Weight loss, excessive exercise, stress | Hypothalamic suppression | |
| Physical examination | Thyromegaly, weight gain, edema | Hypothyroidism |
| Thyroid tenderness, tachycardia, weight loss, velvety skin | Hyperthyroidism | |
| Bruising, jaundice, hepatomegaly | Liver disease | |
| Enlarged uterus | Pregnancy, leiomyoma, uterine cancer | |
| Firm, fixed uterus | Uterine cancer | |
| Adnexal mass | Ovarian tumor, ectopic pregnancy, cyst | |
| Uterine tenderness, cervical motion tenderness | PID, endometritis | |
| Laboratory tests | Beta-subunit human chorionic gonadotropin | Pregnancy |
| Complete blood count with platelet count and coagulation studies | Coagulopathy | |
| Liver function tests, prothrombin time | Liver disease | |
| Thyroid-stimulating hormone | Hypothyroidism, hyperthyroidism | |
| Prolactin | Pituitary adenoma | |
| Blood glucose | Diabetes mellitus | |
| DHEA-S, free testosterone, 173-hydroxyprogesterone if hyperandrogenic | Ovarian or adrenal tumor | |
| Papanicolaou smear | Cervical dysplasia | |
| Cervical testing for infection | Cervicitis, PID | |
| Imaging and tissue sampling | Endometrial biopsy or dilatation and curettage | Hyperplasia, atypia, or adenocarcinoma |
| Transvaginal ultrasonography | Pregnancy, ovarian or uterine tumors | |
| Saline-infusion sonohysterography | Intracavitary lesions, polyps, submucous fibroids | |
| Hysteroscopy | Intracavitary lesions, polyps, submucous fibroids |
Once pregnancy and iatrogenic causes have been excluded, patients should be evaluated for systemic disorders, particularly thyroid, hematologic, hepatic, adrenal, pituitary, and hypothalamic conditions (Table 2). Menstrual irregularities are associated with both hypothyroidism (23.4 percent of cases) and hyperthyroidism (21.5 percent of cases).10 [Strength of recommendation (SOR) B. Consistent cohort studies] Thyroid function tests may help the physician determine the etiology.
Inherited coagulopathy has been shown to be the underlying cause of abnormal uterine bleeding in 18 percent of white women and 7 percent of black women with menorrhagia.11 These patients may present in adolescence with severe menstrual bleeding or frequent bruising. A complete blood count with platelet count should be obtained. If a coagulation defect is suspected, consultation with a hematologist may be the most cost-effective option in the absence of reasonable screening tests for specific abnormalities.11 Because jaundice and hepatomegaly may suggest underlying acquired coagulopathy, liver function tests should be considered.
The presence of galactorrhea, as determined by the history or physical examination, may indicate underlying hyperprolactinemia, which can cause oligoovulation or eventual amenorrhea. A prolactin level confirms the diagnosis of hyperprolactinemia. Hypothalamic suppression secondary to eating disorders, stress, or excessive exercise may induce anovulation, which sometimes manifests as irregular and heavy menstrual bleeding or amenorrhea.
Genital tract pathology may be associated with intermenstrual, postcoital, and heavy menstrual bleeding.4 Any history of abnormal Papanicolaou (Pap) smears, sexually transmitted disease, gynecologic surgery, trauma, or sexual abuse should be elicited. Uterine fibroids, endometrial polyps, adenomyosis, endometrial hyperplasia and atypia, and endometrial cancer should be excluded.13
The evaluation of postmenarchal women who present with abnormal uterine bleeding includes a pelvic examination, as well as a Pap smear if appropriate, to look for vulvar or vaginal lesions, signs of trauma, and cervical polyps or dysplasia. Cervical dysplasia seldom causes abnormal uterine bleeding, but it may be associated with postcoital bleeding.14 Cervical cultures may be indicated if the patient is at risk for infection or if symptoms of infection are present. A bimanual examination in the postmenarchal woman may reveal tenderness associated with infection, an adnexal mass consistent with an ovarian neoplasm or cyst, or uterine enlargement consistent with fibroids, pregnancy, or a tumor.
Because endometrial abnormalities are present in 31 percent of patients with a Pap result of “atypical glandular cells of undetermined significance, favor endometrial origin,” endometrial biopsy is indicated.15 [SOR B, observational studies] Transvaginal ultrasonography may be useful in delineating the underlying cause of abnormal uterine bleeding that is associated with uterine enlargement or an adnexal mass. Even if the pelvic examination is normal, further evaluation of the endometrium may be required to eliminate less obvious abnormalities.
Dysfunctional uterine bleeding, with both anovulatory and, less commonly, ovulatory4 causes, occurs during the childbearing years. It is a diagnosis of exclusion and is made only after pregnancy, iatrogenic causes, systemic conditions, and obvious genital tract pathology have been ruled out (Figure 1).2,16
Anovulatory dysfunctional uterine bleeding is a disturbance of the hypothalamic-pituitary-ovarian axis that results in irregular, prolonged, and sometimes heavy menstrual bleeding. It may occur immediately after menarche but before maturation of the hypothalamic-pituitary-ovarian axis, or it may occur during perimenopause, when declining estrogen levels fail to regularly stimulate the LH surge and resulting ovulation.
Unopposed estrogen stimulation may lead to endometrial proliferation and hyperplasia. Without sufficient progesterone to stabilize and differentiate the endometrium, this mucous membrane becomes fragile and sloughs irregularly. Estrogen also affects uterine vascular tone, angiogenesis, prostaglandin formation, and endometrial nitric oxide production.4
Ovulatory dysfunctional bleeding may include polymenorrhea, oligomenorrhea, midcycle spotting, and menorrhagia (Table 3).6 (pp575-9) Polymenorrhea, a presumed lutealphase dysfunction, results in shortened cycles (less than 21 days), whereas oligomenorrhea, a prolonged follicular-phase dysfunction, results in lengthened cycles (more than 35 days). Mid-cycle spotting occurs before ovulation as the estrogen levels decline.6 Menorrhagia is regularly occurring heavy menstrual bleeding (more than 80 mL per cycle) and may result from the loss of local endometrial hemostasis.
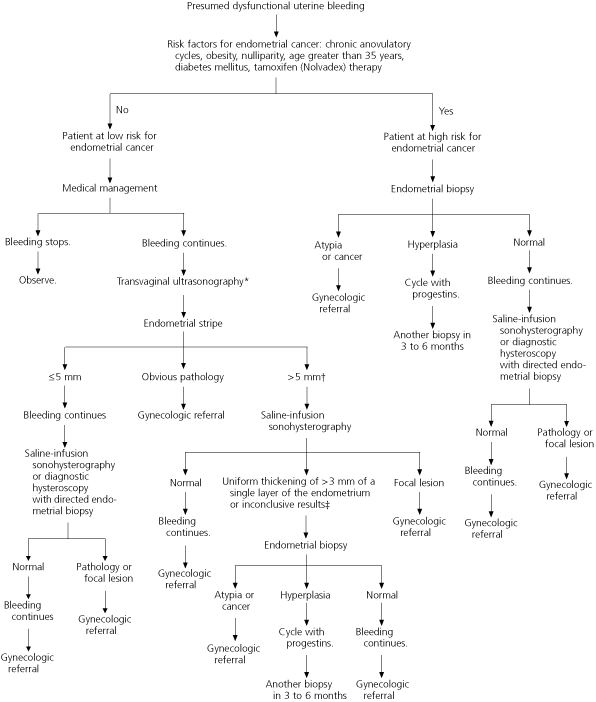
Further Evaluation Based on Risk Factors for Endometrial Cancer
Further evaluation of abnormal uterine bleeding depends on the patient's age and the presence of risk factors for endometrial cancer, which include anovulatory cycles, obesity, nulliparity, age greater than 35 years, and tamoxifen therapy.17,18 Initially, medical management is recommended for premenopausal women at low risk for endometrial carcinoma who are diagnosed with presumed dysfunctional uterine bleeding.
The risk of developing endometrial cancer increases with age.18 The overall incidence of this cancer is 10.2 cases per 100,000 in women aged 19 to 39 years. The incidence more than doubles from 2.8 cases per 100,000 in those aged 30 to 34 years to 6.1 cases per 100,000 in those aged 35 to 39 years. In women aged 40 to 49 years, the incidence of endometrial carcinoma is 36.5 cases per 100,000. Thus, the American College of Obstetricians and Gynecologists recommends endometrial evaluation in women aged 35 years and older who have abnormal uterine bleeding.21 [SOR C, consensus guideline]
Endometrial evaluation (including imaging and tissue sampling) for subtle genital tract pathology is recommended in patients who are at high risk for endometrial cancer and in patients at low risk who continue bleeding abnormally despite medical management.21
| Term | Abnormal uterine bleeding pattern |
|---|---|
| Oligomenorrhea | Bleeding occurs at intervals of > 35 days and usually is caused by a prolonged follicular phase. |
| Polymenorrhea | Bleeding occurs at intervals of < 21 days and may be caused by a lutealphase defect. |
| Menorrhagia | Bleeding occurs at normal intervals (21 to 35 days) but with heavy flow (80 mL) or duration (7 days). |
| Menometrorrhagia | Bleeding occurs at irregular, noncyclic intervals and with heavy flow (80 mL) or duration (7 days). |
| Amenorrhea | Bleeding is absent for 6 months or more in a nonmenopausal woman. |
| Metrorrhagia or bleeding intermenstrual | Irregular bleeding occurs between ovulatory cycles; causes to consider include cervical disease, intrauterine device, endometritis, polyps, submucous myomas, endometrial hyperplasia, and cancer. |
| Midcycle spotting | Spotting occurs just before ovulation, usually because of a decline in the estrogen level. |
| Postmenopausal bleeding | Bleeding recurs in a menopausal woman at least 1 year after cessation of cycles. |
| Acute emergent abnormal uterine bleeding | Bleeding is characterized by significant blood loss that results in hypovolemia (hypotension or tachycardia) or shock. |
| Dysfunctional uterine bleeding | This ovulatory or anovulatory bleeding is diagnosed after the exclusion of pregnancy or pregnancy-related disorders, medications, iatrogenic causes, obvious genital tract pathology, and systemic conditions. |
Imaging and Tissue Sampling
The sensitivity of endometrial biopsy for the detection of endometrial abnormalities has been reported to be as high as 96 percent.22 However, this office-based procedure may miss up to 18 percent of focal lesions,23 including polyps and fibroids, because only a small part of the endometrium may be sampled at any one time. Although endometrial biopsy has high sensitivity for endometrial carcinoma,24,25 its sensitivity for detecting atypical endometrial hyperplasia may be as low as 81 percent.25 [Reference 25: SOR B, meta-analysis of lower quality/inconsistent studies]
Transvaginal ultrasonography may reveal leiomyoma, endometrial thickening, or focal masses. Although this imaging modality may miss endometrial polyps and submucous fibroids, it is highly sensitive for the detection of endometrial cancer (96 percent) and endometrial abnormality (92 percent).26 [SOR A, meta-analysis of consistent, good-quality studies] Compared with dilatation and curettage, endometrial evaluation with transvaginal ultrasonography misses 4 percent more cancers,26,27 but it may be the most cost-effective initial test in women at low risk for endometrial cancer who have abnormal uterine bleeding that does not respond to medical management.28
Saline-infusion sonohysterography bolsters the diagnostic power of transvaginal ultrasonography. This technique entails ultrasound visualization after 5 to 10 mL of sterile saline has been instilled in the endometrial cavity. Its sensitivity and specificity for endometrial cancer are comparable with the high sensitivity and specificity of diagnostic hysteroscopy.29 [SOR B, meta-analysis with significant heterogeneity] Saline-infusion sonohysterography is more accurate than transvaginal ultrasonography in diagnosing intracavitary lesions30,31 and is more accurate than hysteroscopy in diagnosing endometrial hyperplasia.32 The ombination of directed endometrial biopsy and saline-infusion sonohysterography results in a sensitivity of 95 to 97 percent and a specificity of 70 to 98 percent for the identification of endometrial abnormality.33,34 [References 33 and 34: SOR B, diagnostic cohort studies]
Although dilatation and curettage has been the gold standard for diagnosing endometrial cancer,35 it no longer is considered to be therapeutic for abnormal uterine bleeding; furthermore, it is limited in its ability to access the tubal cornua of the uterus.36 Hysteroscopy with biopsy provides more information than dilatation and curettage alone37 and rivals the combination of saline-infusion sonohysterography and endometrial biopsy in its ability to diagnose polyps, submucous fibroids, and other sources of abnormal uterine bleeding.31
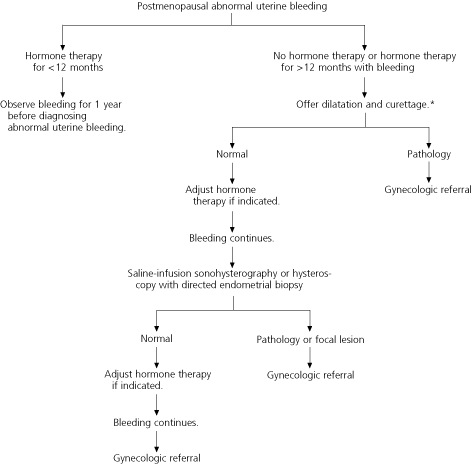
Postmenopausal women with abnormal uterine bleeding, including those who have been receiving hormone therapy for more than 12 months, should be offered dilatation and curettage for evaluation of the endometrium (96 percent sensitivity for the detection of cancer, with a 2 to 6 percent false-negative rate).26 Postmenopausal women who are poor candidates for general anesthesia and those who decline dilatation and curettage may be offered transvaginal ultrasonography or saline-infusion sonohysterography with endometrial biopsy.
Further research is necessary to determine the best method for evaluating the endometrium in patients with abnormal uterine bleeding. However, based on current evidence, saline-infusion sonohysterography with endometrial biopsy appears to provide the most complete evaluation with the least risk33,34 (Figures 223,26,38 and 3).
Medical Management
ANOVULATORY DYSFUNCTIONAL UTERINE BLEEDING
Oral contraceptive pills (OCPs) are used for cycle regulation and contraception. In patients with irregular cycles secondary to chronic anovulation or oligoovulation, OCPs help to prevent the risks associated with prolonged unopposed estrogen stimulation of the endometrium. OCPs effectively manage anovulatory bleeding in premenopausal and perimenopausal women. Treatment with cyclic progestins for five to 12 days per month is preferred when OCP use is contraindicated, such as in smokers over age 35 and women at risk for thromboembolism21 (Table 4).16,39,40
OVULATORY DYSFUNCTIONAL UTERINE BLEEDING
Medical therapy for menorrhagia primarily includes nonsteroidal anti-inflammatory drugs (NSAIDs) and the levonorgestrel releasing intrauterine system (Mirena). The U.S. Food and Drug Administration has approved the use of mefenamic acid (Ponstel), an NSAID, for the treatment for menorrhagia; this agent is well tolerated.41 [SOR A, meta-analysis] The levonorgestrel contraceptive device has been shown to decrease menstrual blood loss significantly and to be superior to cyclic progestins for this purpose.42 [SOR A, meta-analysis]
Although the effect of OCPs on menorrhagia has not been well studied, one small randomized trial comparing OCPs, mefenamic acid, naproxen, and danazol showed no significant difference in their effectiveness in treating menorrhagia.43 [SOR B, single randomized controlled trial] Side effects and cost limit the use of androgens such as danazol and gonadotropin-releasing hormone agonists in the treatment of menorrhagia, but these agents may be used for short-term endometrial thinning before ablation is performed.44 [SOR A, meta-analysis]
| Surgical procedure | Reason for surgery |
|---|---|
| Operative hysteroscopy | Intracavitary structural abnormalities |
| Myomectomy (abdominal, laparoscopic, hysteroscopic) | Leiomyoma |
| Transcervical endometrial resection | Treatment-resistant menorrhagia or menometrorrhagia |
| Endometrial ablation (using various energy systems, principally thermal balloon or rollerball) | Treatment-resistant menorrhagia or menometrorrhagia; secondarily for management of treatment-resistant acute uterine hemorrhage |
| Uterine artery embolization | Leiomyoma |
| Hysterectomy | Atypical hyperplasia, endometrial cancer, or bleeding that does not respond to less invasive uterus-sparing surgeries |
Antifibrinolytics significantly reduce heavy menstrual bleeding. However, these agents are used infrequently because of concerns about safety (i.e., potential for thromboembolism).45
Intravenous administration of conjugated estrogens (Premarin) may be required in women with acute uterine hemorrhage.40 [SOR B, single randomized controlled study]
Surgical Management
When medical therapy fails or is contraindicated, surgical intervention may be required. Hysterectomy is the treatment of choice when adenocarcinoma is diagnosed, and this procedure also should be considered when biopsy specimens contain atypia.13 Hysterectomy and various uterus-sparing surgical procedures for the treatment of abnormal uterine bleeding are beyond the scope of this article but are listed in Table 5.