
Am Fam Physician. 2004;69(11):2628-2635
Sialorrhea (drooling or excessive salivation) is a common problem in neurologically impaired children (i.e., those with mental retardation or cerebral palsy) and in adults who have Parkinson's disease or have had a stroke. It is most commonly caused by poor oral and facial muscle control. Contributing factors may include hypersecretion of saliva, dental malocclusion, postural problems, and an inability to recognize salivary spill. Sialorrhea causes a range of physical and psychosocial complications, including perioral chapping, dehydration, odor, and social stigmatization, that can be devastating for patients and their families. Treatment of sialorrhea is best managed by a clinical team that includes primary health care providers, speech pathologists, occupational therapists, dentists, orthodontists, neurologists, and otolaryngologists. Treatment options range from conservative (i.e., observation, postural changes, biofeedback) to more aggressive measures such as medication, radiation, and surgical therapy. Anticholinergic medications, such as glycopyrrolate and scopolamine, are effective in reducing drooling, but their use may be limited by side effects. The injection of botulinum toxin type A into the parotid and submandibular glands is safe and effective in controlling drooling, but the effects fade in several months, and repeat injections are necessary. Surgical intervention, including salivary gland excision, salivary duct ligation, and duct rerouting, provides the most effective and permanent treatment of significant sialorrhea and can greatly improve the quality of life of patients and their families or caregivers.
Saliva is secreted by the six major salivary glands (two parotid, two submandibular, and two sublingual) and several hundred minor salivary glands. The major salivary glands produce 90 percent of the approximately 1.5 L of saliva that are secreted per day. In the unstimulated (basal) state, 70 percent of saliva is secreted by the submandibular and sublingual glands. When stimulated, salivary flow increases by five times, with the parotid glands providing the preponderance of the saliva.1
The various functions of saliva include mechanical cleansing of the mouth, contributing to oral homeostasis, and helping to regulate oral pH. Saliva also has bacteriostatic and bacteriocidal properties that contribute to dental health and decrease oral odor. It is important in the lubrication of food boluses, and the amylase in saliva begins the digestion of starches.
The parasympathetic nervous system innervates the parotid, submandibular, and sublingual glands with fibers that originate in the pons and medulla, and synapse in the otic and submandibular ganglia. Postganglionic fibers from the otic ganglion provide secretory function to the parotid gland, and fibers from the submandibular ganglion supply secretory function to the submandibular and sublingual glands. The flow of saliva is enhanced by sympathetic innervation, which promotes contraction of muscle fibers around the salivary ducts.
Sialorrhea (drooling or excessive salivation) is defined as saliva beyond the margin of the lip. This condition is normal in infants but usually stops by 15 to 18 months of age. Sialorrhea after four years of age generally is considered to be pathologic.
Physical and psychosocial complications of sialorrhea range from mild and inconvenient symptoms to severe problems that can have a significant negative impact on quality of life. Physical complications include perioral chapping and maceration with secondary infection, dehydration, and foul odor. The psychosocial complications include isolation, barriers to education (such as an inability to share books or computer keyboards), and increased dependency and level of care. Caretakers and loved ones may find it more difficult to demonstrate affection with affected patients, contributing to a potentially devastating stigmatization.
Etiology
Sialorrhea usually is caused by neuromuscular dysfunction, hypersecretion, sensory dysfunction, or anatomic (motor) dysfunction. The most common cause is neuromuscular dysfunction. In children, mental retardation and cerebral palsy are commonly implicated; in adults, Parkinson's disease is the most common etiology. Pseudobulbar palsy, bulbar palsy, and stroke are less common causes (Table 1).
Hypersecretion commonly is caused by inflammation, such as teething, dental caries, and oral cavity infection. Other causes of hypersecretion include side effects from medications (i.e., tranquilizers, anticonvulsants), gastroesophageal reflux, toxin exposure (i.e., mercury vapor), and rabies.
| Neuromuscular/sensory dysfunction | |
| Mental retardation | |
| Cerebral palsy | |
| Parkinson's disease | |
| Pseudobulbar* | |
| Bulbar palsy* | |
| Stroke* | |
| Hypersecretion † | |
| Inflammation (teething, dental caries, oral-cavity infection, rabies) | |
| Medication side effects (tranquilizers, anticonvulsants) | |
| Gastroesophageal reflux | |
| Toxin exposure (mercury vapor) | |
| Anatomic ‡ | |
| Macroglossia (enlarged tongue) | |
| Oral incompetence | |
| Dental malocclusion | |
| Orthodontic problems | |
| Head and neck surgical defects (i.e., “Andy Gump” deformity) | |
Under normal circumstances, persons are able to compensate for increased salivation by swallowing. However, sensory dysfunction may decrease a person's ability to recognize drooling, and anatomic or motor dysfunction may impede the ability to manage increased secretions.
Anatomic abnormalities are usually not the sole cause of drooling but commonly exacerbate other causative conditions. Macroglossia (enlarged tongue) and oral incompetence may predispose patients to salivary spill. Unfortunately, neither of these conditions is easily remedied. Malocclusion and other orthodontic problems may compound oral incompetence; orthodontic correction can reduce sialorrhea.
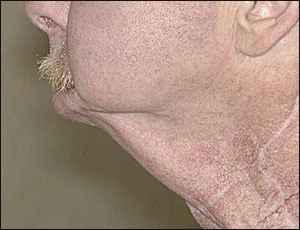
Surgical defects following major head and neck resection and reconstruction also may cause sialorrhea. The most notable example of these anatomic defects is “Andy Gump” deformity, which is caused by the loss of the anterior mandibular arch (Figure 1).
Assessment of Sialorrhea
Objective and subjective measures have been developed to quantify sialorrhea. The objective tests using radioisotope scanning and collection cups strapped to the patient's chin are used primarily for research purposes. A variety of subjective scales for sialorrhea have been described.2 One system rates the severity of drooling on a five-point scale and the frequency of drooling on a four-point scale (Table 2).3 Although scales are useful in assessing and monitoring therapy, the impact of sialorrhea on the patient's quality of life is the most important factor in determining the necessity of therapy.
| Drooling | Points | |
|---|---|---|
| Severity | ||
| Dry (never drools) | 1 | |
| Mild (wet lips only) | 2 | |
| Moderate (wet lips and chin) | 3 | |
| Severe (clothing becomes damp) | 4 | |
| Profuse (clothing, hands, tray, objects become wet) | 5 | |
| Frequency | ||
| Never drools | 1 | |
| Occasionally drools | 2 | |
| Frequently drools | 3 | |
| Constantly drools | 4 | |
Management
Treatment of sialorrhea is best accomplished by using a team approach.4 The primary care physician usually focuses on the complete history and physical examination of the patient, with special attention to the impact of drooling on quality of life and the potential for improvement. Speech pathologists and occupational therapists work with patients to improve their swallowing mechanics and to support their posture with devices such as the head-back wheelchair. Dentists and orthodontists assess and treat dental and oral diseases and malocclusion. Otolaryngologists identify and correct causes of aerodigestive obstruction like macroglossia and adenotonsillar hypertrophy that contribute to drooling. Neurologists, otolaryngologists, and primary care physicians can assess the patient for significant cranial neuropathies.
After a thorough assessment, a consensus on appropriate treatment options should be developed by the treatment team, the patient, and the patient's family. Treatments can be offered in a stepwise fashion, from least invasive, nonsurgical therapies to most invasive.
For minimal problems, in children under four years of age, or in adults with unstable neurologic function, observation is frequently the best option.4 Minimal problems also can be treated with a feeding program aimed at improving oromotor control, although this effort is rarely successful.
Any situational factors should be corrected, and dental malocclusion and caries should be treated. Adenotonsillectomy should be performed, when indicated, and patients should be fitted with appropriate wheelchairs and braces, if necessary.
Several orthodontic appliances may be used for the treatment of sialorrhea. Customized plates formed to fit the palate can aid in better lip closure.5,6 Movable beads can be placed on the upper plate; they stimulate tongue movement, thus helping to deflect saliva toward the pharynx. The use of these beads in combination with swallowing therapy have been successful in patients with moderate sialorrhea.7–9
Biofeedback and automatic cueing techniques have successfully treated patients with mild neurologic dysfunction and drooling. One study10 showed that biofeedback was successful in patients older than eight years who had mild to moderate problems. Patients are trained to associate a behavior with a cue; for example, swallowing or wiping the face is associated with an electronic beep. These devices can be used for several hours a day. The drawback to these devices is that patients become habituated to the stimulus, and the devices become less effective after repetitive use.11
Positive and negative reinforcement has been described as an adjunct in the management of sialorrhea in patients with moderate neurologic disease. Caretakers praise patients for not drooling or require them to wipe their faces when they forget to swallow.12
In a small, prospective study,13 acupuncture improved sialorrhea based on subjective measures in seven of 10 patients. Over a six-week period, patients were treated 30 times with needles placed in five locations in the tongue. Further study of the effectiveness of acupuncture in the treatment of sialorrhea is warranted.
If sialorrhea continues to interfere with the patient's health and quality of life after non-invasive measures have been tried, medication, radiation, and surgical therapy should be considered.
ANTICHOLINERGIC MEDICATIONS
Anticholinergic medications block the parasympathetic innervation of the salivary glands. Several studies14–17 have demonstrated the effectiveness of glycopyrrolate and scopolamine (Transderm Scop) in the treatment of sialorrhea (Table 3).14–18 Unfortunately, even these relatively selective anticholinergic medications have side effect profiles that rise in proportion to their efficacy.
Glycopyrrolate is best known for its drying properties and a limited central nervous system activity. Prospective, randomized trials14,15 of the use of this agent in the treatment of sialorrhea have demonstrated a significant reduction in drooling; however, approximately 20 percent of patients stopped taking the medicine because of side effects, and 23 percent experienced behavior changes.14,15 Transdermal scopolamine, applied as a patch behind the ear, was well tolerated in short-term studies,16,17 but its use was limited by side effects of urinary retention and blurred vision.
| Agent | How supplied | Dosage | Side effects | Cost* |
|---|---|---|---|---|
| Glycopyrrolate | Scored tablets,† 1 or 2 mg | Adults: Start at 0.5 mg orally, one to three times daily; titrate to effectiveness and tolerability‡ | Constipation, excessive oral dryness, urinary retention, blurred vision, hyperactivity, irritability | $ 0.66 per 1-mg tablet |
| Children: 0.04 mg per kg per dose orally, two to three times daily; titrate to effectiveness and tolerability | ||||
| Scopolamine (Transderm Scop) | Patch, 1.5 mg | Apply patch every day | Pruritus at patch site, urinary retention, irritability, blurred vision, dizziness, glaucoma | 20.99 per four 1.5-mg patches |
| Botulinum toxin A | Vial, 100 U per vial | Under ultrasound guidance, injections of 10 to 40 units into each submandibular and parotid gland | Pain at injection site, excessive oral dryness | 521.25 per vial |
Anticholinergics are contraindicated in patients with glaucoma, obstructive uropathy, gastrointestinal motility disorders, and myasthenia gravis. Also, these medications often are poorly tolerated in elderly patients who have multiple comorbidities.
BOTULINUM TOXIN
Intraglandular injection of botulinum toxin type A recently has been reported to improve sialorrhea18 (Table 3).14–18 Under ultrasound guidance, botulinum toxin type A was injected into the bilateral parotid and submandibular glands of 10 adult patients. Nine of the patients improved, and no patient had complications. Treatment response lasted approximately five months, making repeat treatments necessary for long-term control.
GASTROESOPHAGEAL REFLUX CONTROL
Many developmentally delayed or neurologically impaired patients who have sialorrhea also have significant gastroesophageal reflux. It has been postulated that controlling reflux will reduce drooling; however, this conjecture has not been confirmed by research, and it is unlikely that control of reflux has any clinically significant effect on sialorrhea.19
RADIATION THERAPY
Radiation to the salivary glands is a reasonable treatment option in elderly patients who are not candidates for surgery and cannot tolerate medical therapy.20 Radiation produces xerostomia that may last months to years. The dose may be titrated to reach the desired effect, and treatment can be repeated as necessary. Malignancies induced by radiation therapy typically do not occur until 10 to 15 years after treatment and, therefore, are less of a concern in patients who are elderly and debilitated.20
SURGICAL OPTIONS
Surgical options in the treatment of sialorrhea include surgery on the salivary glands and ducts, and surgery to denervate the glands (Table 4). Surgery to denervate the salivary glands is performed through the middle ear, where the tympanic plexus and chorda tympani travel before entering the major salivary glands. The procedure is relatively simple and fast, and does not require general anesthesia. This surgery has few side effects, and patients typically do not complain of loss of taste. Unfortunately, salivary function returns within six to 18 months, when nerve fibers regenerate.21
| Surgical therapy | Advantages | Disadvantages |
|---|---|---|
| Submandibular duct relocation | No external scar | Duct relocation is an uncommon procedure |
| Low incidence of ranula with sublingual gland excision | ||
| Potential for anterior dental caries | ||
| Without sublingual gland excision, patient may develop ranula | ||
| Potential for aspiration | ||
| Submandibular gland excision | Very good control of sialorrhea | External scar |
| Commonly performed procedure | Potential for dental caries | |
| Parotid duct relocation | Redirects flow in the stimulated state | Risk of sialocele |
| Potential for aspiration | ||
| Relocation is uncommon procedure | ||
| Parotid duct ligation | Simple, fast procedure | Risk of sialocele |
| Decreases flow in the stimulated state | ||
| Transtympanic neurectomy | Technically easy, fast procedure | Predictable return of salivary function Requires multiple procedures |
| Does not require general anesthesia | ||
| Useful in elderly patients |
The most definitive treatment of sialorrhea is surgery to excise the major salivary glands or to ligate or reroute the major salivary ducts. This procedure typically involves a combination parotid duct ligation or rerouting with either submandibular gland excision or duct rerouting. Sublingual gland excision is suggested if the submandibular ducts are rerouted to prevent formation of salivary retention cysts.22,23 Preservation of salivation with reduction of drooling has been demonstrated following rerouting of the parotid and submandibular ducts to the posterior oropharynx, and rerouting procedures spare patients external scars and the risk of facial nerve injury.24–26
The most definitive surgical procedure, which includes bilateral parotid duct ligation and submandibular gland excision, is highly successful, with nearly total elimination of sialorrhea, a low incidence of facial weakness, and significant patient and caretaker satisfaction.27 Although this is the most invasive of treatment options, the severity of sialorrhea may be sufficient to require such an aggressive therapy.