
Am Fam Physician. 2004;69(12):2853-2860
The spondyloarthropathies include ankylosing spondylitis, reactive arthritis (including Reiter’s syndrome), psoriatic arthritis, inflammatory bowel disease–associated spondyloarthropathy, and undifferentiated spondyloarthropathy. These diseases are linked by their association with the HLA-B27 gene and by the presence of enthesitis as the basic pathologic lesion. Additional clinical features include inflammatory back pain, dactylitis, and extra-articular manifestations such as uveitis and skin rash. The history and physical examination are the major diagnostic tools, although radiographic evidence of sacroiliitis is helpful. Therapeutic options include non-steroidal anti-inflammatory drugs, sulfasalazine, methotrexate, and tumor necrosis factor-α inhibitors. Early recognition and appropriate treatment can help to limit disability.
The spondyloarthropathies are a diverse group of inflammatory arthritides that share certain genetic predisposing factors and clinical features1 (Table 1).1–3 Their most characteristic feature is inflammatory back pain.4 Enthesitis, another characteristic feature, involves inflammation at sites where tendons, ligaments, or joint capsules attach to bone (Table 2). Enthesitis is believed to be the primary lesion in the spondyloarthropathies, whereas synovitis is the main lesion in rheumatoid arthritis. Dactylitis (inflammation of an entire digit), commonly termed “sausage digit,” also occurs in the spondyloarthropathies and is thought to arise from joint and tenosynovial inflammation.
Although diagnostic criteria for the spondyloarthropathies have been developed for research purposes, the criteria rarely are used in clinical practice. Diagnosis is based primarily on the history and physical examination. There are no specific diagnostic tests for spondyloarthropathies. Supporting laboratory findings include absence of rheumatoid factor, elevation of the erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level, and presence of anemia of chronic disease. HLA-B27 testing is of limited value. The synovial fluid typically is inflammatory (more than 2,000 white blood cells per mL, with a predominance of neutrophils), but this finding is nonspecific. Evidence of sacroiliitis or spondylitis may be seen on radiographs of the pelvis and lumbar spine.
Although the spondyloarthropathies are grouped together, they display distinct clinical features. It is likely that an interplay among genetic, environmental, and immunologic factors is responsible for the various clinical manifestations of these diseases. Infection with an unknown organism or exposure to an unknown antigen in a genetically susceptible patient (HLA-B27–positive) is hypothesized to result in the clinical expression of a spondyloarthropathy.5 This article reviews the diagnosis and treatment of the most common spondyloarthropathies.
Ankylosing Spondylitis
The prevalence of ankylosing spondylitis, the most common spondyloarthropathy, is 0.1 to 0.2 percent in the general U.S. population (possibly as high as 1 percent in certain groups) and is related to the prevalence of HLA-B27. Ankylosing spondylitis most often affects white males between 15 and 40 years of age.6
The inflammatory back pain in ankylosing spondylitis typically has an insidious onset and a dull quality, and the pain radiates into the gluteal regions. Back pain is worse in the morning, improves with activity, and has a nocturnal component.6
Over time, axial arthritis can progress from the sacroiliac joints, gradually ascending to involve the cervical spine. Limited spinal mobility results from spinal deformities such as flattening of the lumbar lordosis, exaggeration of the thoracic kyphosis, and hyperextension of the cervical spine.
| Features | Ankylosing spondylitis | Reactive arthritis (including Reiter’s syndrome) | Psoriatic arthritis | IBD-associated spondyloarthropathy | ||
|---|---|---|---|---|---|---|
| Prevalence | 0.1% to 0.2% | 0.1% | 0.2% to 0.4% | Rare | ||
| Age at onset | Late teens to early adulthood | Late teens to early adulthood | 35 to 45 years | Any age | ||
| Male-to-female ratio | 3:1 | 5:1 | 1:1 | 1:1 | ||
| HLA-B27 | 90% to 95% | 80% | 40% | 30% | ||
| Sacroiliitis | ||||||
| Frequency | 100% | 40% to 60% | 40% | 20% | ||
| Distribution | Symmetric | Asymmetric | Asymmetric | Symmetric | ||
| Syndesmophytes | Delicate, marginal | Bulky, nonmarginal | Bulky, nonmarginal | Delicate, marginal | ||
| Peripheral arthritis | ||||||
| Frequency | Occasional | Common | Common | Common | ||
| Distribution | Asymmetric, lower limbs | Asymmetric, lower limbs | Asymmetric, any joint | Asymmetric, lower limbs | ||
| Enthesitis | Common | Very common | Very common | Occasional | ||
| Dactylitis | Uncommon | Common | Common | Uncommon | ||
| Skin lesions | None | Circinate balanitis, keratoderma blennorrhagicum | Psoriasis | Erythema nodosum, pyoderma gangrenosum | ||
| Nail changes | None | Onycholysis | Pitting, onycholysis | Clubbing | ||
| Ocular conditions | Acute anterior uveitis | Acute anterior uveitis, conjunctivitis | Chronic uveitis | Chronic uveitis | ||
| Oral conditions | Ulcers | Ulcers | Ulcers | Ulcers | ||
| Cardiac conditions | Aortic regurgitation, conduction defects | Aortic regurgitation, conduction defects | Aortic regurgitation, conduction defects | Aortic regurgitation | ||
| Pulmonary features | Upper lobe fibrosis | None | None | None | ||
| Gastrointestinal conditions | None | Diarrhea | None | Crohn’s disease, ulcerative colitis | ||
| Renal conditions | Amyloidosis, IgA nephropathy | Amyloidosis | Amyloidosis | Nephrolithiasis | ||
| Genitourinary conditions | Prostatitis | Urethritis, cervicitis | None | None | ||
Although Schober’s test is nonspecific, it is useful for measuring spinal mobility. The test is performed by marking the patient’s back over the L5 spinous process (between the posterior superior iliac spines) and 10 cm above this point. The patient then is asked to bend forward. The distance between the two marks should increase by 5 cm or more in normal persons. An increase of less than 5 cm suggests decreased range of motion of the lumbar spine.
Some patients with ankylosing spondylitis develop arthritis in the hips and shoulders, frequently early in the course of the disease. Other peripheral joints usually become affected later. The lower extremities most often are involved in an asymmetric fashion.
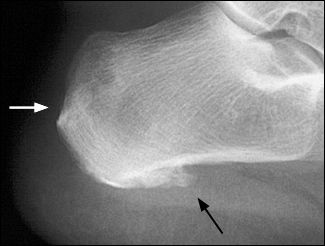
Enthesitis is common in patients with ankylosing spondylitis. Inflammation at the Achilles tendon and plantar fascia calcaneal insertions is particularly common and manifests as heel pain. Like arthritis, enthesitis typically is aggravated by rest and improved with activity.
Extra-articular features of ankylosing spondylitis can involve almost any organ system. Constitutional symptoms include fatigue, anorexia, and mild fever. Anterior uveitis is the most frequent extra-articular manifestation, occurring in 25 to 30 percent of patients.7 The uveitis usually is acute, unilateral, and recurrent. Eye pain, red eye, blurry vision, photophobia, and increased lacrimation are presenting signs. Cardiac manifestations include aortic and mitral root dilatation, with regurgitation and conduction defects. Fibrosis may develop in the upper lobes of the lungs in patients with longstanding disease.
| Achilles tendon insertion on the calcaneus |
| Plantar fascia insertion on the calcaneus |
| Patellar tendon insertion on the tibial tubercle |
| Superior and inferior aspects of the patella |
| Metatarsal heads |
| Base of the fifth metatarsal |
| Spinal ligament insertions on the vertebral bodies |
Although there is no laboratory test to diagnose ankylosing spondylitis, the HLA-B27 gene has been found to be present in about 90 to 95 percent of affected white patients in central Europe and North America.5 However, a positive HLA-B27 assay is nonspecific, because the antigen is found in 8 to 10 percent of white persons and in up to 2 percent of black persons.5 Furthermore, only 1 to 2 percent of HLA-B27–positive persons develop ankylosing spondylitis.5 The ESR rate and CRP level are elevated in 50 to 70 percent of patients, but the elevations generally do not correlate with disease activity.7
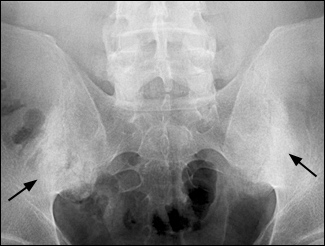
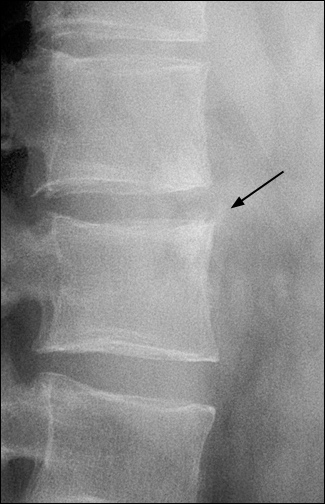
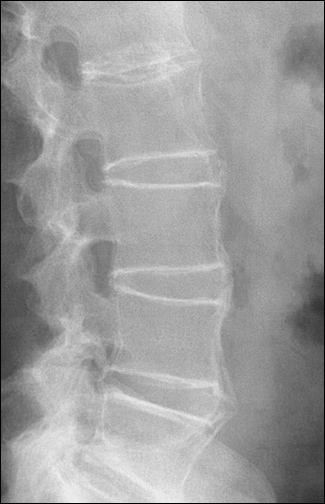
Radiographic features of ankylosing spondylitis include bilateral symmetric sacroiliitis, with initial sclerosis progressing to erosive changes and total ankylosis or fusion of the sacroiliac joints (Figure 1). Spinal involvement because of enthesitis initially is seen as squaring of the vertebral bodies, then osteitis at the vertebral margins (Figure 2) and, eventually, ossification of the annulus fibrosus, with the formation of delicate marginal syndesmophytes in a gradually ascending pattern that occasionally results in the classic “bamboo spine” (Figure 3). Syndesmophytes are due to ossification of the annulus fibrosus, which eventually may bridge the intervertebral space.
The diagnosis of ankylosing spondylitis should be suspected in young patients who present with inflammatory back pain. The disease is diagnosed less frequently in women, because axial symptoms are less severe. Peripheral arthritis may be more prominent in women.
Pharmacologic treatment of ankylosing spondylitis begins with nonsteroidal anti-inflammatory drugs (NSAIDs), which have been shown to provide rapid relief of inflammatory back pain. A positive response to NSAID therapy is helpful in diagnosing ankylosing spondylitis.8
Second-line agents such as sulfasalazine (Azulfidine) are used when patients do not respond to NSAIDs or are unable to tolerate these agents. One meta-analysis demonstrated that sulfasalazine is safe and effective in the short-term treatment of ankylosing spondylitis.9 The meta-analysis of five randomized double-blind, placebo-controlled trials (RCTs)7 showed statistically significant and clinically important improvement in patients treated with sulfasalazine, although two subsequent trials failed to show efficacy for the drug.10,11 Sulfasalazine appears to be effective in alleviating peripheral arthritis in patients with ankylosing spondylitis but is less effective in patients with axial disease.
Although not well studied, methotrexate (Rheumatrex) may be beneficial in patients with prominent peripheral arthritis.12 Oral corticosteroids in conventional dosages are of little value in the treatment of ankylosing spondylitis, but intra-articular corticosteroid injections can provide rapid and sustained relief in isolated inflamed joints.8 Intravenously administered pamidronate (Aredia) appears to have a modest effect on disease activity.13
Several trials14–16 have evaluated the efficacy of the tumor necrosis factor-α (TNF-α) inhibitors etanercept (Enbrel) and infliximab (Remicade) in patients with ankylosing spondylitis. Data from the trials clearly indicate that these agents are effective in treating the inflammatory symptoms of ankylosing spondylitis. The TNF-α inhibitors also have potential as disease-modifying agents.17,18 The U.S. Food and Drug Administration has approved etanercept for use in the treatment of ankylosing spondylitis.
Nonpharmacologic therapy is an important adjunct to drug therapy. Interventions include patient education, outpatient physical therapy, a home exercise program (including spinal extension exercises), and proper posturing. Inpatient rehabilitation may be necessary in selected patients.
Reactive Arthritis and Reiter’s Syndrome
Reactive arthritis is an aseptic arthritis that is triggered by an infectious agent located outside the joint. Reiter’s syndrome, one of the earliest described forms of reactive arthritis, is a clinical triad of nongonococcal urethritis, conjunctivitis, and arthritis.
Reactive arthritis usually begins one to four weeks after a genitourinary or gastrointestinal tract infection. Causative organisms include (among others) Chlamydia, Ureaplasma, Shigella, Salmonella, Yersinia, and Campylobacter species.19
The arthritis tends to be oligoarticular, preferentially affecting the joints of the lower extremities. Onset is typically acute: within a few days, two to four joints become painful and swollen in an asymmetric distribution. Weight loss and temperatures of up to 38.8°C (102°F) have been recorded during the acute phase.19 Enthesitis is common, especially in the heel. Dactylitis and inflammatory back pain also are common.
Extra-articular manifestations are essential in supporting the diagnosis of reactive arthritis. Conjunctivitis is present in up to 50 percent of patients and can develop at any time during the course of the disease. This feature is reported to be more common in patients with reactive arthritis subsequent to genitourinary or Shigella infection. As in ankylosing spondylitis, uveitis most often is acute, unilateral, and recurrent.20,21
Urethritis classically is a sterile variant, although Chlamydia trachomatis and Ureaplasma urealyticum have been isolated in many patients with reactive arthritis. The urethritis can range from asymptomatic to severe with accompanying prostatitis. In women with reactive arthritis, cervicitis usually is marked by vaginal discharge.
Acute diarrhea frequently is a presenting manifestation when reactive arthritis develops after Shigella, Yersinia, or Salmonella infection. The diarrhea precedes the appearance of musculoskeletal symptoms by up to one month.19
Oral ulcers are common and may be painless. Circinate balanitis, a painless erythematous lesion of the glans penis, is present in about 20 percent of male patients with reactive arthritis.21
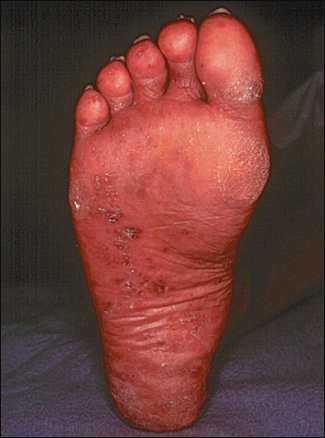
Keratoderma blennorrhagicum, a manifestation of Reiter’s syndrome, is characterized by the development of hyperkeratotic lesions on the palms of the hands or the soles of the feet (Figure 5). Lesions often begin as clear vesicles on an erythematous base and progress to macules, papules, and nodules. The lesions are indistinguishable from those of pustular psoriasis.19
Although reactive arthritis usually has a self-limited course of three to 12 months, symptomatic treatment is warranted. Up to 50 percent of patients have recurrent bouts of arthritis, and 15 to 30 percent develop chronic arthritis or sacroiliitis.20 Treatment begins with NSAIDs. Sulfasalazine has been shown to be effective in patients with chronic reactive arthritis.22 Intra-articular corticosteroid injections can be effective in controlling disease in individual joints.
An association between certain spondyloarthropathies (reactive arthritis, psoriatic arthritis, undifferentiated spondyloarthropathy) and human immunodeficiency virus infection has been noted, particularly in sub-Saharan Africa.25 The patients have tended to have more severe disease, and the increased prevalence is thought to be due to poor availability of effective antiretroviral therapy.
Psoriatic Arthritis
Psoriasis affects up to 1 to 2 percent of the general U.S. population, and psoriatic arthritis has been reported in as many as 20 percent of patients with psoriasis. In the majority of cases, skin manifestations precede joint involvement, although the reverse occurs in 15 to 20 percent of cases.26
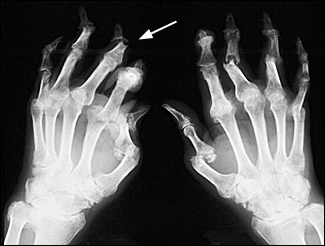
There are five recognized patterns of psoriatic arthritis: an oligoarticular type (four or fewer joints affected); a polyarticular type (five or more joints affected); a pattern with predominant distal interphalangeal (DIP) joint involvement; arthritis mutilans; and psoriatic spondylitis. The oligoarticular pattern accounts for more than 70 percent of cases27 (Figure 6).
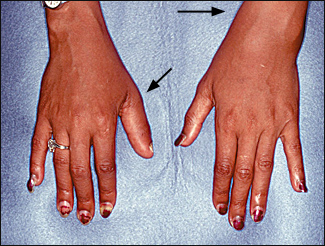
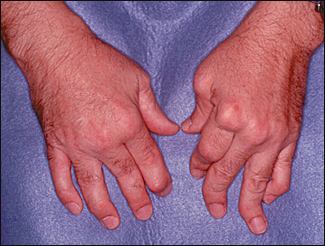
Psoriatic arthritis usually is asymmetric, and distal joints often are affected. These features distinguish psoriatic arthritis from rheumatoid arthritis. Clinical presentations range from mild, remitting arthritis to highly destructive disease (Figure 7). The severity of the arthritis usually does not correlate with the extent of skin involvement.
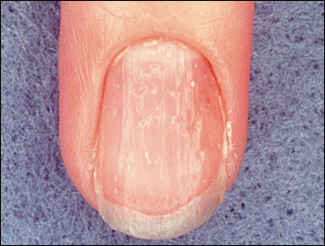
If a diagnosis of psoriatic arthritis is being considered, the skin should be examined carefully for psoriatic lesions. In addition to manifesting in typical sites (e.g., extensor surface of the knee), psoriasis can be present as a small patch in the scalp, ears, anal cleft, perineum, or umbilicus. Nail lesions, including pitting and onycholysis, occur in more 80 percent of patients with psoriatic arthritis27 (Figure 8). In psoriatic arthritis, uveitis tends to be chronic and can occur bilaterally.
Radiographs in patients with psoriatic arthritis demonstrate an erosive arthritis, with frequent DIP joint involvement and pencil-in-cup changes because of marked resorption of bone (Figure 9). Other findings, including enthesitis with periosteal reaction, sacroiliitis, and spondylitis, are similar to those in patients with reactive arthritis.
Treatment of psoriatic arthritis is directed at both the skin and joint manifestations. A variety of topical therapies, including corticosteroids, retinoids, and ultraviolet-light therapy, can be used for the skin disease. NSAID therapy, the initial treatment for joint manifestations, improves swelling and tenderness. Oral corticosteroid therapy occasionally is useful in the treatment of generalized disease, and intraarticular corticosteroid injections can control localized joint disease. Second-line agents include methotrexate, sulfa-salazine, cyclosporine (Sandimmune), and TNF-α inhibitors.1,28,29 Etanercept has been shown to control disease activity and inhibit progression of joint destruction.30
Spondyloarthropathy Associated with Inflammatory Bowel Disease
Spondyloarthropathy occurs in up to 20 percent of patients who have inflammatory bowel disease (IBD).2 The association occurs more often in patients with Crohn’s disease than in those with ulcerative colitis. In some patients, arthritis manifests before clinical bowel disease.
Typically, the arthritis affects the lower extremities in an asymmetric fashion. Onset usually is abrupt, and the arthritis has a migratory pattern. The arthritis generally subsides in six to eight weeks, although recurrence is common, and 10 percent of patients develop chronic arthritis. In up to 20 percent of affected patients, IBD-associated spondyloarthropathy manifests as a spondylitis that is indistinguishable from idiopathic ankylosing spondylitis. Exacerbations of peripheral arthritis and IBD tend to coincide, whereas axial disease is independent of IBD activity.2
Extra-articular manifestations of IBD-associated spondyloarthropathy include uveitis, which is usually bilateral, and chronic skin lesions, such as erythema nodosum and pyoderma gangrenosum. Less common features include clubbing, periostitis, amyloidosis, and granulomatous disease of bone and joint.
The treatment of IBD-associated arthritis is slightly more complex than the treatment of other spondyloarthropathies. NSAIDs should be used cautiously, because they can exacerbate the bowel disease.2 Sulfasalazine has been effective in the treatment of IBD and arthritis.31 Data on azathioprine (Imuran) and methotrexate therapy in patients with severe disease also have been promising.2 Treatment with TNF-α inhibitors may have a beneficial effect on the associated arthritis in patients with IBD.3
Undifferentiated Spondyloarthropathy
The term “undifferentiated spondyloarthropathy” is used to describe manifestations of a spondyloarthropathy in patients who do not meet criteria for any of the well-defined spondyloarthropathies. Over time, a small proportion of these patients develop a well-defined spondyloarthropathy. However, most patients have less specific symptoms, including inflammatory back pain, unilateral or alternating buttock pain, enthesitis, dactylitis and, occasionally, extra-articular manifestations.
Patients with undifferentiated spondyloarthropathy generally have a good prognosis and often respond well to NSAID therapy. Treatment of patients with more severe disease is similar to that for ankylosing spondylitis.
Further information on the spondyloarthropathies is available on the following Web sites:http://www.arthritis.org,http://www.rheumatology.org, andhttp://www.spondylitis.org.
| Key clinical recommendations | Strength of recommendation | References |
|---|---|---|
| Clinical criteria supported by laboratory tests, synovial fluid analysis, and radiographs help to establish the presence of spondyloarthropathies. | C | 4,5,7 |
| Initial management begins with NSAIDs | C | 8 |
| Sulfasalazine (Azulfidine) may be an effective second-line agent that provides short-term relief. | B | 9–11 |
| Early trials indicate that tumor necrosis factor-α inhibitors such as etanercept (Enbrel) and infliximab (Remicade) are effective in treating inflammatory symptoms. | B | 17,18 |
| Despite the possibility of a bacterial etiology in reactive arthritis, antibiotic therapy has been ineffective. | B | 23 |
| Second-line treatment of psoriatic arthritis (after NSAIDs have failed) includes systemic corticosteroids, methotrexate (Rheumatrex), sulfasalazine, cyclosporine (Sandimmune), and tumor necrosis factor-α inhibitors. | B | 1,28–30 |