
A more recent article on pretravel consultation is available.
Am Fam Physician. 2004;70(1):89-99
Advising travelers on vaccine-preventable illnesses is increasingly becoming the responsibility of primary care physicians. The approach to vaccine recommendations should be based on a thorough assessment of the risks for travel-related diseases, the time available before trip departure, and current knowledge of the epidemiology of vaccine-preventable diseases. Routine childhood vaccinations should be reviewed in all travelers and updated as necessary. Yellow fever vaccination may be required for entry by countries that lie within a yellow fever zone or for travelers coming from an endemic area to prevent introduction of the disease. Immunization against hepatitis B virus should be considered in travelers who expect to have close contact with local populations that have high rates of hepatitis B transmission. Japanese encephalitis vaccine should be offered to travelers who plan prolonged trips to rural areas in southeast Asia or the Indian subcontinent during the transmission season. Typhoid fever immunization is recommended for travelers who may be exposed to potentially contaminated food and drink. Preexposure rabies vaccination should be considered in travelers who plan a prolonged duration of stay in a remote area or who engage in activities that might involve working near animals or that could attract animals. Physicians should be aware of the adverse events and contraindications associated with each travel vaccine.
As international travel to exotic locations becomes increasingly common, it is necessary for more physicians to maintain familiarity with current recommendations for travel health safety. Immunizations and preventive medicines are key parts of travel preparation, and careful attention to them can reduce the risks of infections acquired while abroad. Travel vaccines generally fall into one of three categories: (1) routine immunizations typically administered during childhood that should be updated or boosted, (2) legally required immunizations necessary for entry into certain countries, and (3) recommended immunizations that may be useful, depending on the risks of exposure at the travel destination.1–3 Vaccines are not available for all travel-related infections (e.g., malaria). In these cases, preventive medication may be necessary to keep the traveler healthy.
Advising travelers on vaccine- and medication-preventable diseases is increasingly becoming the responsibility of primary care physicians. The approach to travel health recommendations should be based on an assessment of the risks for travel-related illnesses, the time available before trip departure, and the current epidemiology of preventable diseases. Physicians should take into account the adverse events and contraindications associated with each vaccine and medication. This article reviews the overall approach to travel immunizations and provides an overview of the immunizations that are recommended or required for international travel (Table 1). Information about preventive medication has appeared previously in American Family Physician.4
| Vaccine | Standard regimen | Booster | Age | Adverse effects | Cost per dose* | Notes |
|---|---|---|---|---|---|---|
| Hepatitis A | ||||||
| Havrix | 1.0 mL IM | 1.0 mL IM 6 to 12 months after first dose | ≥ 19 years† | Injection site soreness, headaches | $64 | Safety in pregnancy not determined |
| Vaqta | 1.0 mL IM | 1.0 mL IM 6 to 12 months after first dose | ≥ 19 years† | Injection site soreness, headaches | $78 | |
| Hepatitis B | ||||||
| Recombivax-HB | 1.0 mL IM at 0, 1, and 6 months | A booster is not routine | ≥ 20 years† | Injection site soreness, headaches | $74 | Safety in pregnancy not determined Contraindicated in yeast hypersensitivity |
| Engerix-B | 1.0 mL IM at 0, 1, and 6 months (Accelerated schedule: 1.0 mL IM at 0, 1, 2, and 12 months) | 1.0 mL IM 12 months after first dose | ≥ 20 years† | Injection site soreness, headaches | $60 | |
| Combined hepatitis A and hepatitis B | ||||||
| Twinrix | 1.0 mL IM at 0, 1, and 6 months | Not routine | ≥ 18 years | Injection site soreness, headaches, nausea | $94 | Safety in pregnancy not determined Contraindicated in yeast hypersensitivity |
| Immune globulin (human) | ||||||
| Baygam | 0.02 to 0.06 mL per kg | 0.02 to 0.06 mL per kg every 3 to 5 months | ≥ 2 years | Injection site soreness, urticaria | $31 for 2 mL | Safety in pregnancy not determined Never shown to transmit hepatitis B, hepatitis C, or human immunodeficiency virus Avoid concurrent administration with MMR and varicella vaccines |
| Japanese encephalitis | ||||||
| Je-Vax | 1.0 mL SQ on days 0, 7, 30 | 1.0 mL SQ every 2 to 3 years | ≥ 3 years | Fever, headache, nausea, vomiting (urticaria and angioedema are rare) | $312 for 3 doses | Safety in pregnancy not determined Administer last dose 10 days before trip departure |
| Meningococcal | ||||||
| Menomune | 0.5 mL SQ | 0.5 mL SQ every 3 to 5 years | ≥ 2 years | Injection site soreness | $80 | Safe in pregnancy |
| Rabies | ||||||
| RabAvert | 1.0 mL IM on days 0, 7, 21, or 28 | Every 2 to 5 years (check serum for antibody presence) | All ages | Myalgias, lymphadenopathy | $155 | Give only if risk of exposure to rabies is substantial; pregnancy is not a contraindication to preexposure therapy After animal bite, rabies vaccine on day of bite and on day 3 required if preexposure rabies vaccine administered (rabies immune globulin not necessary) Mefloquine or chloroquine may interfere with immune response to intradermal Imovax vaccine if administered concurrently |
| Imovax | 1.0 mL IM on days 0, 7, 21, or 28 | Every 2 to 5 years (check serum for antibody presence) | All ages | Myalgias, lymphadenopathy | $160.59 | |
| Typhoid fever | ||||||
| Vivotif Berna (oral Ty21a) | Four capsules: 1 capsule given every other day | Four-capsule regimen every 5 years | ≥ 6 years | Nausea, vomiting, cramping, fever | $43 for 4 capsules | Oral Ty21a vaccine capsules must be kept refrigerated; each capsule should be taken whole (do not chew) with cool liquid one hour before a meal Oral Ty21a vaccine contraindicated in pregnancy andimmunocompromised patients Start mefloquine (Lariam) or chloroquine (Aralen) at least 3 days after completion of oral Ty21a vaccine Safety of Typhim VI vaccine in pregnancy not determined |
| Typhim VI | 0.5 mL IM | 0.5 mL IM every 2 years | ≥ 2 years | Nausea, vomiting, cramping | $49 | |
| Yellow fever | ||||||
| YF-Vax | 0.5 mL SQ | 0.5 mL SQ every 10 years | ≥ 9 months | Headaches, myalgias, fever, encephalitis (rarely in the elderly) | $62 | Consider carefully in elderly patients Contraindicated in pregnancy, patients with egg allergy, and immunocompromised patients Avoid concurrent administration with other live virus vaccines (MMR, oral polio, varicella, oral Ty21a) |
Risk Assessment
Immunizations should be recommended according to the patient’s risk of travel-related diseases and not solely according to geographic destination. A number of resources provide updated information about risks to travelers (Table 2).5 To properly assess a traveler’s risk of illness, the physician first should consider the details of the planned journey5–7 : the exact itinerary, including all geographic destinations and possible stopovers; duration of stay in each location; type of lodging (urban or rural, hotel or tent); planned activities (animal contact, river- or lake-water exposure, eating habits); seasonal risks (time of year); and level of anticipated contact with local residents.
| Web sites |
| American Society of Tropical Medicine and Hygiene: http://www.astmh.org |
| Centers for Disease Control and Prevention, Travelers’ Health Information: http://www.cdc.gov/travel |
| International Association for Medical Assistance to Travelers: http://www.iamat.org |
| International Society of Travel Medicine: http://www.istm.org |
| MD Travel Health: http://www.mdtravelhealth.com |
| Pan American Health Organization: http://www.paho.org |
| Shoreland’s Travel Health Online: http://www.tripprep.com |
| U.S. Department of State, Bureau of Consular Affairs: http://www.travel.state.gov (202-647-5225) |
| World Health Organization, International Travel and Health: http://www.who.int/ith |
| Publications |
| Cook GC, Zumla AI, eds. Manson’s Tropical diseases. 21st ed. London: Saunders, 2003. |
| DuPont HL, Steffen R. Textbook of travel medicine and health. 2d ed. Lewiston, N.Y.: B.C. Decker, 2001. |
| Health Information for International Travel, 2003–2004. Atlanta: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Infectious Diseases, Division of Quarantine, 2003. |
| Jong EC, McMullen R. The travel and tropical medicine manual. 3d ed. Philadelphia: Saunders, 2003. |
Physicians then should review the status of the traveler’s general health, focusing on underlying diseases that may have implications during the trip.6 Previous immunizations, allergies to medications and vaccine components (especially eggs), and current medications also should be reviewed.5–7 The physician should make a special effort to identify travelers who are at particularly high risk for travel-related illnesses (Table 3).3,6 An overall approach to vaccination of travelers based on risk assessment is presented in Figure 1 and Table 4.
| Persons who backpack or trek while traveling |
| Persons older than 65 years |
| Persons living in the United States but born in another country and who travel back to country of origin |
| Persons who are immunocompromised |
| Persons with human immunodeficiency virus infection |
| Persons who have received an organ transplant |
| Persons who use immunosuppressive medications |
| Persons who travel on a long-term basis |
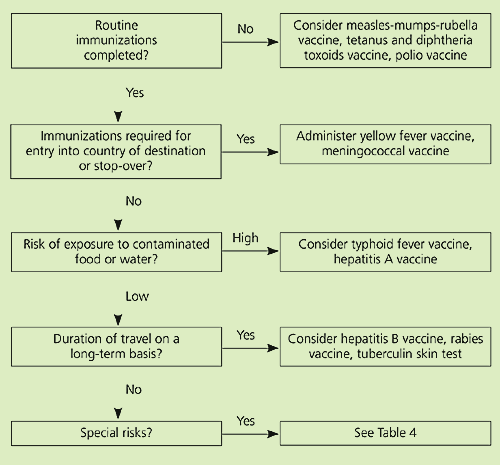
| Intervention | Risk factor |
|---|---|
| Consider hepatitis B vaccine | Travel to area where hepatitis B is endemic May need medical care while abroad Person living in the United States but born in another country and who travels back to country of origin |
| Consider influenza vaccine | Travel during influenza season (November through March in northern hemisphere, April through September in southern hemisphere) |
| Consider Japanese encephalitis vaccine | Travel to rural areas of Indian subcontinent or southeast Asia |
| Consider polio vaccine | Travel to country where polio is endemic (e.g., India, Nigeria, Pakistan, Egypt, Afghanistan, Niger, and Somalia) Age > 65 years |
| Consider pneumococcal vaccine | Presence of cardiopulmonary disease, asplenia, cirrhosis, diabetes mellitus Planned prolonged stay in remote area Planned work near animals or activity that could attract animals |
| Consider rabies vaccine | Unable to report animal bite (e.g., young age, disability, remote location) |
Travelers, particularly those going to developing countries, should be encouraged to seek medical advice early in their planning (at least four weeks in advance). Consultation with a travel clinic may be helpful if the destination is high risk. The amount of time remaining before departure determines whether the standard schedule for a primary immunization series can be used or whether an accelerated schedule, if one exists, should be offered.2 When departure is imminent and an accelerated vaccine schedule is used, vaccine efficacy may not be maximal by the time of departure, and this fact must be discussed with the patient.2
Physicians who provide consultations to travelers should base their recommendations on the current epidemiology of vaccine-preventable diseases at each destination. The Centers for Disease Control and Prevention (CDC) publication, “Health Information for International Travel,” is one of the standard references for travel immunization recommendations and is updated regularly.8 Additional information may be obtained online from the CDC (http://www.cdc.gov/travel) and the World Health Organization (WHO) (http://www.who.int/ith).
Routine Immunizations
Travel provides an opportunity for the physician to review and update a patient’s routine immunizations.1,6 Travelers to areas where postexposure tetanus immunization might be unavailable should consider receiving a booster dose of tetanus and diphtheria (Td) toxoids before departure if five or more years have elapsed since their last vaccination.9
Measles is endemic in many developing nations, and a booster of measles-mumps-rubella (MMR) vaccine is warranted for any person born after 1956 who does not have documentation of two doses of the vaccine or immunity by serum antibody testing.10 Children six to 11 months of age should receive one dose of MMR vaccine if traveling to highly endemic areas, but they still must receive two doses of the vaccine after 12 months of age to be considered fully immunized.10
Polio is a good example of the need for physicians to keep current with changing epidemiology. Intensive immunization campaigns have resulted in a marked decrease in polio throughout the world. Polio remains endemic in seven countries: India, Nigeria, Pakistan, Egypt, Afghanistan, Niger, and Somalia.11 Travelers to these countries are advised to receive a single booster of inactivated polio vaccine (IPOL) if the primary doses have already been administered.
Varicella (chickenpox) immunity should be reviewed and, if needed, children one through 12 years of age should receive a single dose of vaccine (Varivax), while those 13 years and older should receive two doses of vaccine administered four to eight weeks apart.1,2,8,12,13 In particular, this vaccine should be considered for women of child-bearing age who do not have documented varicella disease before vaccination or antibody titers.
Finally, the influenza vaccine is recommended for all international travelers during influenza season. While influenza typically occurs from November until March in the northern hemisphere, the incidence of the disease peaks from April until September in the southern hemisphere. Patients should receive the most current vaccine available.
Required Immunizations
YELLOW FEVER
Yellow fever is a rare but potentially fatal viral infection that is endemic in equatorial Africa (Figure 2) and South America (Figure 3) , where the virus is transmitted by day-biting mosquito vectors. The clinical presentation of the disease ranges from a mild febrile illness to a life-threatening disease characterized by hepatitis, renal failure, hemorrhagic fever, and shock.
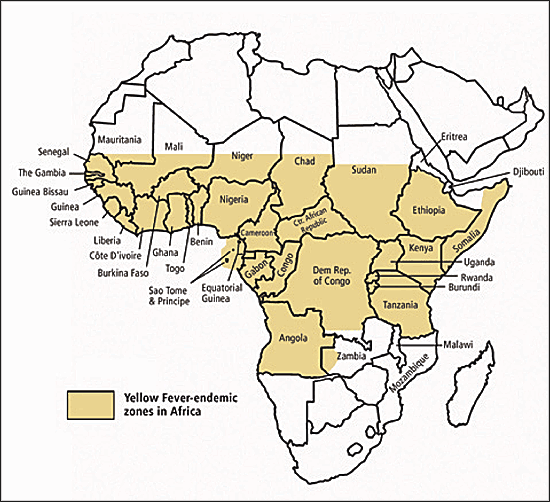
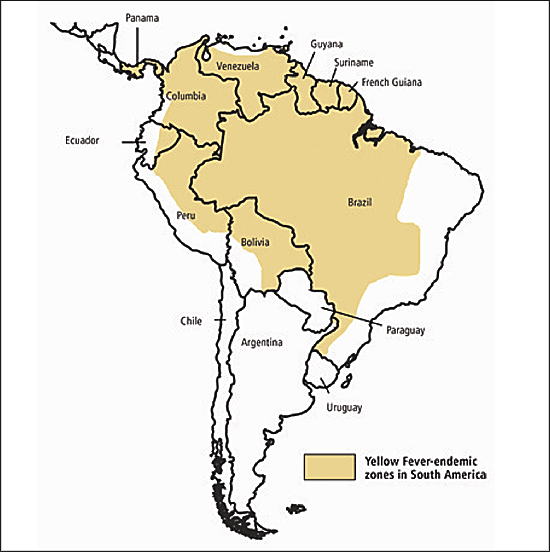
Yellow fever vaccination is recommended for patients older than nine months who are traveling to areas where yellow fever is reported.15 It also is recommended for travelers to rural areas of countries that do not officially report yellow fever but are within the endemic zone.15 Many yellow-fever endemic countries require proof of vaccination for entry. Other countries may require proof of vaccination if a person is traveling from an endemic area to prevent introduction of the disease. Yellow fever vaccination may be required even if the person merely passes through an endemic region while traveling to the final destination. Physicians can obtain country-specific requirements for yellow fever vaccination from the CDC.8
The yellow fever vaccine (YF-Vax) is a live-attenuated virus preparation delivered in a single subcutaneous inoculation of 0.5 mL. It induces neutralizing antibodies in 99 percent of recipients within 30 days of receipt.16 Immunity is likely to be lifelong, but revaccination is required at 10-year intervals.15 For purposes of international travel, the vaccine must be administered at an approved yellow fever vaccination center. Proof of immunization should be documented on an Official International Certificate of Vaccination Against Yellow Fever, which becomes valid 10 days after vaccination to meet entry and exit requirements for all countries. Yellow fever immunization is usually available at local health departments, which are approved vaccination centers.
Reactions to the yellow fever vaccine are generally mild, but analysis of vaccine recipients in the United States from 1990 to 1998 found that persons 65 years or older were at an increased risk for neurologic and systemic reactions.17 [Evidence level B, case series] Thus, its use should be considered carefully in this population.
Yellow fever vaccination is not recommended in pregnancy, and pregnant women who are not immune to yellow fever should delay their travel to any high-transmission area until after delivery. If the travel itinerary of a pregnant woman does not present a substantial risk, and immunization is required only for entry, the physician should provide the woman with a waiver letter.8,15 [Evidence level C, consensus/expert guidelines] Pregnant women who must travel to areas with active transmission should be vaccinated because the small risk to the mother and fetus from the vaccine is believed to be outweighed by the risk of yellow fever.15 [Evidence level C, consensus/ expert guidelines]
Recommended Immunizations
HEPATITIS A
The inactivated hepatitis A virus vaccines (Havrix, Vaqta) are recommended for all international travelers except those going to destinations in North America (except Mexico), western Europe, Japan, Australia, and New Zealand.8,18,19 Travelers preferably should receive a single intramuscular dose of 1.0 mL four weeks before departure. Vaccination two weeks before travel still may be useful because up to 94 percent of patients develop protective antibodies within two weeks of the first dose.19 [Evidence level A, randomized controlled trial (RCT)] A 1.0-mL booster given six to 12 months later can provide protective antibody levels for at least 10 years.19 Both vaccines provide protective antibody levels in 94 to 100 percent of patients within four weeks of vaccination.20,21 [References 20 and 21—Evidence level A, RCTs] The safety of the vaccine in pregnant women has not been determined.
Travelers who need optimal hepatitis A protection earlier than two weeks after the first dose of hepatitis A vaccine should receive immune globulin with the first vaccine dose but at a different injection site.8,19,22 Those who receive vaccination less than two weeks before departure and who do not receive immune globulin are still at risk of infection, so administration of immune globulin should be considered.8,19 [Evidence level C, consensus/expert opinion] Simultaneous receipt of hepatitis A vaccine and immune globulin results in lower antibody titers than occur when only hepatitis A vaccine is given, but protective antibody levels exceed those achieved when immune globulin is given alone.2,23 Immune globulin also should be offered to travelers who are allergic to the vaccine, younger than two years, or pregnant.19 It is given by intramuscular injection and can provide protection in 85 to 90 percent of patients for three to five months, depending on the dose used (0.02 mL per kg or 0.06 mL per kg).19
HEPATITIS B
While childhood vaccination against hepatitis B now is routine in the United States, many adult travelers have never been immunized.24 Hepatitis B vaccination should be considered for patients who have a potential for close contact with a local population that has a high rate of hepatitis B transmission, patients planning an extended stay (six months or longer) in an area where hepatitis B is endemic (e.g., South America, Africa, southeast Asia, South Pacific), those with a potential need for medical treatment while abroad, and those born overseas who are traveling back to their country of origin.
The standard schedule for administering the hepatitis B vaccine (Recombivax-HB, Engerix-B) in adults 20 years and older calls for three doses of vaccine (each 1.0 mL) at zero, one, and six months. An accelerated schedule with Engerix-B consists of vaccination at zero, one, and two months, with a booster given 12 months after the first dose.8,25 The vaccine is not contraindicated in pregnancy.8
COMBINED HEPATITIS A AND B
A combination hepatitis A and B vaccine (Twinrix) containing the same antigenic components as Engerix-B and pediatric Havrix is available for use in adults older than 18 years. It is as efficacious as each of the monovalent vaccines.25,26 Primary immunization occurs at zero, one, and six months. An accelerated schedule of zero, one, and three weeks, with a fourth dose 12 months after the first dose, is as efficacious as the standard schedule.26 Its safety in pregnancy has not been determined.
JAPANESE ENCEPHALITIS
Japanese encephalitis virus, an arboviral infection transmitted by day-biting mosquitoes, is prevalent in the Indian subcontinent, China, Korea, Japan, and other southeast Asian countries.27 The majority of human cases are asymptomatic, but the virus can cause severe encephalitis with residual neuropsychiatric sequelae.
Japanese encephalitis vaccine (Je-Vax) should be offered to patients who plan to remain for 30 days or longer in endemic areas during the transmission season, particularly if travel destinations might include rural areas. Vaccination also should be considered for short-term travelers who engage in extensive outdoor activities or visit areas of epidemic transmission.
Primary immunization in patients three years or older consists of three doses of 1.0 mL, each given by subcutaneous injection on days zero, seven, and 30. An accelerated schedule, in which doses are given on days zero, seven, and 14, can be used when departure is imminent.27 The vaccine’s efficacy is 91 percent after two doses.28 [Evidence level A, RCT] A booster dose may be given three years after the primary series if continued exposure in high-risk areas is expected. Because generalized urticaria and angioedema of the face, lips, or oropharynx occasionally have occurred up to two weeks after immunization, the last dose of vaccine should be administered at least 10 days before trip departure.27,29
The safety of the vaccine in pregnancy has not been determined. Pregnant women who must travel to an area where the risk of Japanese encephalitis is high should be vaccinated when it is thought that the risks of immunization are outweighed by the risk of infection to the mother and fetus.27 [Evidence level C, consensus/expert guidelines]
TYPHOID FEVER
Typhoid fever immunization is recommended for travelers going to highly endemic areas in Central and South America, the Indian subcontinent, and Africa.30 It also is recommended for travelers who may be exposed to potentially contaminated food and drink, such as those journeying beyond the usual tourist routes.30 Typhoid vaccines (Vivotif Berna, a live-attenuated oral Ty21a vaccine, and Typhim VI) are approximately 50 to 80 percent effective and cannot substitute for careful selection of food and drink.
Primary vaccination with oral Ty21a consists of one enteric-coated capsule taken on alternate days for four doses.30 Vaccine-elicited immunity occurs 14 days after receipt of the last vaccine dose, with an overall efficacy of approximately 50 to 80 percent.30 [Evidence level A, RCT] A booster dose, consisting of the entire four-capsule regimen, is recommended every five years for those at continued risk.30 The most common adverse effect reported is mild gastrointestinal upset. The vaccine is contraindicated in pregnant women, children under the age of six years, and immunocompromised patients. Care must be taken if this vaccine is given in association with antibiotics because they may kill the live-attenuated organisms.
Primary vaccination with Typhim VI in patients two years or older consists of a single 0.5-mL dose given intramuscularly. Protective immunity is elicited 14 days after vaccine receipt.30 The efficacy of this vaccine has been reported to be 50 to 80 percent.8,30 [Evidence level A, RCT] A booster dose given every two years is recommended for continued exposure. No data have been reported regarding its use in pregnant women or immunocompromised patients, although it theoretically is a safer alternative in these groups.
MENINGOCOCCAL
Meningococcal vaccine (Menomune) is recommended for travelers to sub-Saharan Africa, where epidemics of serogroups A or C meningococcal disease occur frequently from December through June in the “meningitis belt” from Senegal to Ethiopia31 (Figure 4) . The vaccine is required for pilgrims to Saudi Arabia during the Hajj and at other religious holidays.8
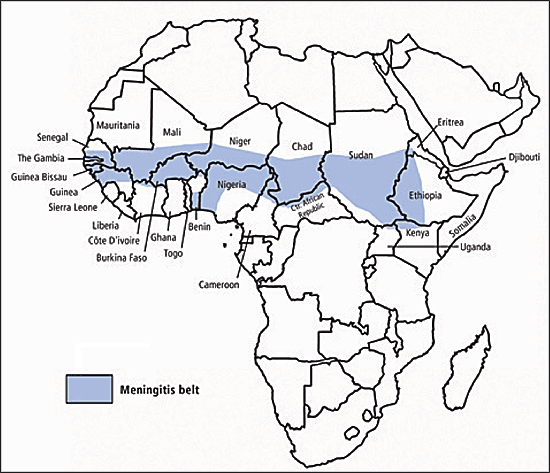
The vaccine is effective only against sero-groups A, C, Y, and W-135.25,31 Primary immunization in patients two years and older consists of a single 0.5-mL dose given by subcutaneous injection, and this dose confers immunity for at least three years.31 Protective levels of antibody are achieved in seven to 10 days.8 Vaccination is not contra-indicated in pregnancy.31 Revaccination may be considered within three to five years for continued exposure.31
RABIES
Canine rabies remains endemic in the Indian subcontinent, China, southeast Asia, the Philippines, parts of Indonesia, Latin America, Africa, and countries of the former Soviet Union.1,2,32 Postexposure prophylaxis, although effective, may not be readily available.33 Preexposure rabies vaccination should be considered for travelers who plan a prolonged stay (more than 30 days) in an endemic region, who travel in remote areas, work near animals, engage in activities that could attract animals (e.g., hiking, cycling), or for persons who cannot report an exposure if bitten (e.g., young children).
In the United States, intramuscular formulations of the purified chick embryo cell vaccine (RabAvert) and human diploid cell vaccine (Imovax) are available. Preexposure rabies immunization consists of three 1.0-mL doses of one of the rabies vaccine formulations given on days zero, seven, and 21 or 28.34 After a high-risk bite, travelers who received preexposure vaccination still require local wound care and two additional rabies vaccine doses (on the day of the bite and on day 3), but administration of rabies immune globulin is not necessary.33