
Am Fam Physician. 2004;70(7):1293-1300
Osteoporosis is characterized by low bone mineral density and a deterioration in the microarchitecture of bone that increases its susceptibility to fracture. The World Health Organization defines osteoporosis as a bone mineral density that is 2.5 standard deviations or more below the reference mean for healthy, young white women. The prevalence of osteoporosis in black women is one half that in white and Hispanic women. In white women 50 years and older, the risk of osteoporotic fracture is nearly 40 percent over their remaining lifetime. Of the drugs that have been approved for the prevention or treatment of osteoporosis, the bisphosphonates (risedronate and alendronate) are most effective in reducing the risk of vertebral and nonvertebral fractures. Risedronate has been shown to reduce fracture risk within one year in postmenopausal women with osteoporosis and in patients with glucocorticoid-induced osteoporosis. Hormone therapy reduces fracture risk, but the benefits may not outweigh the reported risks. Teriparatide, a recombinant human parathyroid hormone, reduces the risk of new fractures and is indicated for use in patients with severe osteoporosis. Raloxifene has been shown to lower the incidence of vertebral fractures in women with osteoporosis. Salmon calcitonin is reserved for use in patients who cannot tolerate bisphosphonates or hormone therapy.
Osteoporosis is a disease that is characterized by low bone mass and a deterioration in the microarchitecture of bone that increases its susceptibility to fracture.1 Normal bone mineral density (BMD) measured using dual x-ray absorptiometry is a T-score that falls within 1 standard deviation (SD) of the reference mean for healthy, young white women. Based on epidemiologic studies, the World Health Organization (WHO) defines osteoporosis as a BMD (hip, spine, or wrist) that is 2.5 SDs or more below the reference mean for healthy, young white women (corresponding to a T-score below −2.5) and defines osteopenia as a BMD that is between 1 and 2.5 SDs below the reference mean.2
| Key Clinical Recommendations | Label | References |
|---|---|---|
| BMD screening is recommended for women 65 years of age and older without risk factors for osteoporosis. | C | 17 |
| BMD screening should begin at age 60 years in women who are at increased risk for osteoporotic fractures. | C | 17 |
| The bisphosphonates risedronate (Actonel) and alendronate (Fosamax) are the most effective agents for reducing the risk of vertebral and nonvertebral fractures in women with postmenopausal osteoporosis | A | 19,21–23 |
| Patients who are beginning long-term treatment with prednisone (more than three months in a dosage of 5 mg per day or higher) or an equivalent also should receive a bisphosphonate as well as calcium and vitamin D supplementation, regardless of their T-score. | C | 33 |
| Although hormone therapy reduces fracture risk, the benefits may be outweighed by the reported risks of treatment. | A | 38 |
Men generally have 20 percent greater BMD than women. Blacks have 20 percent greater bone density than whites. Therefore, neither men nor blacks are affected with osteoporosis as frequently as white women, although they can develop the disease. Glucocorticoids can induce osteoporosis in any of these groups.
Impact of Osteoporosis
Osteoporosis is responsible for almost 1 million vertebral and hip fractures annually (Figure 1).6 In 1995, osteoporotic fractures resulted in 2.5 million physician visits, 432,000 hospitalizations, and 180,000 nursing home admissions.7 In the United States alone, annual medical expenditures for the management of osteoporotic fractures may be as high as $15 billion.1
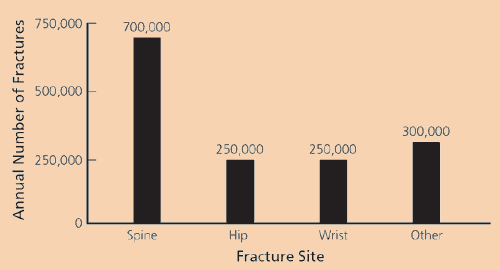
Vertebral fractures trigger back pain, limit activity, and confine patients to bed. Multiple vertebral fractures cause kyphosis and loss of height. Fracture at any site increases the risk for subsequent fracture8: up to 20 percent of women who have an incident vertebral fracture incur another fracture within one year.9 One analysis10 found that postmenopausal women with hip or clinical (i.e., symptomatic) vertebral fractures had an age-adjusted increased risk of death (greater than sixfold risk [6.68] after hip fracture, greater than eightfold risk [8.64] after vertebral fracture) during the next four years.
Risk Factors and Screening
Reported risk factors for osteoporosis include a family history of the disease, hormonal dysfunction, sedentary lifestyle, low body weight, smoking, alcohol abuse, and calcium and vitamin D deficiencies.11 Glucocorticoid therapy also leads to fracture in up to 50 percent of patients12; bone loss is related to the dosage and duration of therapy, and proceeds rapidly during the first six months of treatment.13
A recent review14 found that the relative risk of osteoporosis is highest in women who are menopausal, undergo oophorectomy before the age of 45, have a grandmother with hip fracture, have diabetes mellitus, currently smoke, use alcohol heavily, or have decreased weight-bearing activity because of physical disability.
Based on research conducted to date, it appears that the short-term risk for osteoporotic fractures can be estimated by BMD testing and identification of risk factors. Low body weight (less than 70 kg [154 lb]) is the best predictor of low BMD.15 Note, however, that the instruments (e.g., questionnaires) used to assess clinical risk factors for low BMD or fractures have moderate to high sensitivity but low specificity,14 and that the role of clinical risk factors in deciding which patients to treat remains unclear.
Low BMD increases the risk of fractures. At the 12-month follow-up in the National Osteoporosis Risk Assessment,16 postmenopausal women 50 years and older with no previous diagnosis of osteoporosis but a T-score of −2.5 or lower had an adjusted fracture risk that was 2.74 times higher than the risk in women with a normal BMD.
The U.S. Preventive Services Task Force (USPSTF)17 recommends BMD screening for women 65 years and older without risk factors. Screening should begin at 60 years in women who are at increased risk for osteoporotic fractures. The USPSTF makes no recommendation for or against BMD screening in postmenopausal women who are younger than 60 years or women aged 60 to 64 years who are not at increased risk for fractures.
Options for Prevention and Treatment
Because complications of osteoporosis progress quickly after fracture, rapidly effective therapy is required to reduce fracture risk. A National Osteoporosis Foundation guide offers useful treatment recommendations.18
The U.S. Food and Drug Administration (FDA) has approved a number of agents for use in the prevention or treatment of osteoporosis (Table 1). Head-to-head comparisons of the efficacy of these agents in preventing fractures have not been conducted.
| Agents | Dosage | FDA-approved indications | |
|---|---|---|---|
| Bisphosphonates | |||
| Risedronate (Actonel) | 5 mg orally once a day | Prevention or treatment of postmenopausal osteoporosis | |
| 35 mg orally once a week | Treatment of glucocorticoid-induced osteoporosis | ||
| 5 mg orally once a day | |||
| Alendronate (Fosamax) | 5 mg orally once a day | Prevention of postmenopausal osteoporosis | |
| 35 mg orally once a week | |||
| 10 mg orally once a day | Treatment of postmenopausal osteoporosis | ||
| 70 mg orally once a week | |||
| 5 mg orally once a day (10 mg orally once a day in postmenopausal women not receiving estrogen) | Treatment of glucocorticoid-induced osteoporosis | ||
| 10 mg orally once a day | Treatment of osteoporosis in men | ||
| 70 mg orally once a week | |||
| Hormones | |||
| Conjugated equine estrogen | 0.625 mg orally once a day | Prevention of postmenopausal osteoporosis | |
| Various estrogen preparations | Dosage depends on agent. | Prevention of postmenopausal osteoporosis | |
| Recombinant human parathyroid hormone | |||
| Teriparatide (Forteo) | 20 mcg SC once a day | Treatment of postmenopausal osteoporosis | |
| Treatment to increase bone mass in men with osteoporosis who are at high risk for fracture | |||
| Selective estrogen receptor modulator | |||
| Raloxifene (Evista) | 60 mg orally once a day | Prevention or treatment of postmenopausal osteoporosis | |
| Salmon calcitonin (Miacalcin) | 200 IU intranasally once a day (alternating nostrils daily) | Treatment of postmenopausal osteoporosis | |
| 100 units SC or IM every other day | |||
BISPHOSPHONATES
Bisphosphonates suppress osteoclast-mediated bone resorption. Of the FDA-approved agents, bisphosphonates are the most effective in reducing the risk of vertebral and nonvertebral fractures.19,20 Calcium (1,000 to 1,500 mg per day) and vitamin D (400 to 800 IU per day) typically are administered along with bisphosphonates.1
Risedronate (Actonel), alendronate (Fosamax), and ibandronate (Boniva) are approved for the prevention and treatment of osteoporosis. Only risedronate and alendronate currently are available in oral formulations. Both of these agents are indicated for the prevention or treatment of postmenopausal osteoporosis and for the treatment of glucocorticoid-induced osteoporosis. Alendronate also is approved for the treatment of osteoporosis in men. Once-a-week formulations of risedronate and alendronate are available for use in preventing or treating postmeno-pausal osteoporosis.
Because food and certain minerals reduce the absorption of bisphosphonates, risedronate and alendronate should be taken at least 30 minutes before the first food, drink (other than water), or medication of the day. Tablets should be swallowed with 6 to 8 oz of water. To reduce the risk of gastroesophageal irritation, patients should remain upright for at least 30 minutes after dosing.
In randomized clinical trials,19,21–23 risedronate and alendronate substantially reduced the risk of vertebral fractures. In one placebo-controlled trial,20 risedronate significantly lowered the number of hip and other nonvertebral fractures in 70- to 79-year-old women who had severe osteoporosis and an extremely low BMD. In another trial,23 alendronate reduced the risk of hip fractures in women with vertebral fractures and a low BMD.
Administered once a day, risedronate and alendronate were equivalent to placebo in safety.24,25 However, postmarketing reports showed a high incidence of gastroesophageal irritation and ulceration in patients taking alendronate.26–28 Although reported adverse gastrointestinal events with risedronate were low, bisphosphonates have the potential to cause gastrointestinal irritation. Studies29,30 comparing once-a-day and once-a-week formulations of either bisphosphonate demonstrated similar efficacy and tolerability.
Postmenopausal Osteoporosis. Several three-year trials19–21 demonstrated the efficacy of risedronate therapy in women with post-menopausal osteoporosis (Table 2).19–23,31 The two Vertebral Efficacy with Risedronate Therapy (VERT) trials19,21 evaluated the effectiveness of risedronate, compared with placebo, in reducing the risk of fracture in women with postmenopausal osteoporosis. The North American VERT trial19 included patients with two or more vertebral fractures at baseline or one vertebral fracture and a low spinal BMD (T-score of −2 or lower), and the multinational study21 included patients with two or more vertebral fractures at baseline. The Hip Intervention Program (HIP)20 assessed the efficacy of risedronate in reducing hip fractures in elderly women. About 60 percent of the patients in the HIP study were 70 to 79 years of age with established osteoporosis, and about 40 percent were 80 years or older with a low femoral-neck BMD or one or more clinical risk factors for hip fractures.
| Relative reduction of fracture risk for active treatment vs. placebo | Absolute reduction of fracture risk for active treatment (NNT) | |||||
|---|---|---|---|---|---|---|
| Trial | Inclusion criteria (number of patients in the trial) | Dosage | Vertebral fractures | Nonvertebral fractures | Vertebral fractures | Nonvertebral fractures |
| Risedronate (Actonel) | ||||||
| VERT19 | Two or more vertebral fractures or one vertebral fracture and a T-score of −2.0 or lower (2,458) | 5 mg per day | 65% reduction after 1 year (P < .001) | NR | 4% reduction after 1 year (25) | NR |
| 41% reduction after 3 years (P = .003) | 39% reduction after 3 years (P = .02) | 5% reduction after 3 years (20) | 3.2% reduction after 3 years (31) | |||
| VERT21 | Two or more vertebral fractures (1,226) | 5 mg per day | 61% reduction after 1 year (P = .001) | NR | 7.4% reduction after 1 year (14) | NR |
| 49% reduction after 3 years (P < .001) | 33% reduction after 3 years (P = .06) | 10.9% reduction after 3 years (9) | 5.1% reduction after 3 years (20) | |||
| HIP20 | Osteoporosis or one or more risk factors for osteoporosis (9,331) | 2.5 and 5 mg per day | NR | 20% reduction after 3 years (P = .03) | NR | 1.8% reduction after 3 years(56) |
| Alendronate (Fosamax) | ||||||
| FIT22 | Low femoral-neck BMD (4,432) | 5 mg per day for 2 years followed by 10 mg per day for 2 years | 44% reduction after 4 years (P = .001) | 12% reduction after 4 years (P = .13) | 1.7% reduction after 4 years (59) | 1.5% reduction after 4 years (67) |
| FIT23 | Low femoral-neck BMD plus one or more clinical vertebral fractures (2,027) | 5 mg per day for 2 years followed by 10 mg per day for 1 year | 47% reduction after 3 years (P < .001) | 20% reduction after 3 years (P = .063) | 7% reduction after 3 years (14) | 2.8% reduction after 3 years (36) |
| Alendronate Phase III Osteoporosis Treatment Study31 | Osteoporosis (994) | 5 or 10 mg per day for 3 years 20 mg per day for two years followed by 5 mg per day for 1 year | 48% reduction after 3 years, based on pooling of all data (P = .03) | 21% reduction after 3 years (P values not reported; not significant) | 3% reduction after 3 years (33) | 2.2% reduction after 3 years (45) |
In the VERT studies,19,21 risedronate reduced the relative risk of vertebral fractures by up to 65 percent after one year, and the reduction was sustained for the three years of treatment. The risk of nonvertebral fractures was reduced by as much as 20 to 39 percent in the three trials.19–21 In the HIP study,20 rised-ronate significantly reduced the three-year risk of hip fractures by 40 percent (P = .009) in women aged 70 to 79 years with confirmed osteoporosis and by 60 percent (P = .003) in women with confirmed osteoporosis and one vertebral fracture at baseline. The HIP study found no significant reduction in the risk of hip fractures in women 80 years or older who had one or more risk factors but did not have confirmed osteoporosis.
Alendronate has been shown to increase BMD and decrease fracture risk22,23,31 (Table 2).19–23,31 In one study,31 alendronate was used in postmenopausal women with a lumbar-spine T-score of −2.5 or lower. Patients received alendronate in a dosage of 5 or 10 mg per day for three years, alendronate in a dosage of 20 mg per day for two years followed by 5 mg per day for one year, or placebo for three years. Although the 10-mg dosage was more effective than the 5-mg dosage in increasing BMD, it did not provide additional fracture protection. The alendronate regimen of 20 mg per day followed by 5 mg per day was similar in efficacy to the regimen of 10 mg per day in increasing BMD but not in preventing fractures. A significant reduction in new vertebral fractures (48 percent,P = 0.03) was observed only when all of the alendronate data were pooled.
The Fracture Intervention Trial (FIT)22,23 evaluated the efficacy of alendronate in reducing the risk of vertebral and nonvertebral fractures in postmenopausal women with a low femoral-neck BMD. In the three-year arm of the study,23 patients with one or more vertebral fractures at baseline received alendronate in a dosage of 5 mg per day for two years followed by 10 mg per day in the third year, or placebo. In the four-year treatment arm,22 patients with a T-score of −1.6 or lower but no vertebral fracture at baseline received alendronate in a dosage of 5 mg per day for two years followed by 10 mg per day for two years, or placebo. Compared with placebo, alendronate reduced the risk of new vertebral fractures by 47 percent (P < .001) in women in the three-year treatment arm and by 44 percent (P = .001) in the women in the four-year treatment arm. Reductions in the overall risk of nonvertebral fractures did not reach statistical significance.
Prospective one-year data on the reduction of vertebral fracture risk with alendronate therapy are not available for individual clinical trial populations. A pooled subanalysis of the FIT data,32 which included only patients with a femoral-neck T-score of −2.5 or lower but no vertebral fractures or patients with an existing vertebral fracture, demonstrated a 59 percent reduction (P < .001) in the risk of vertebral fractures after 12 months of therapy and a 63 percent reduction (P = .014) in the risk of hip fractures after 18 months of therapy. The greatest risk reductions occurred in the patients with the lowest BMD.
Glucocorticoid-Induced Osteoporosis. The American College of Rheumatology33 recommends that patients who are beginning long-term treatment with prednisone (three months or longer in a dosage of 5 mg per day or higher) or an equivalent also receive a bisphosphonate, as well as calcium and vitamin D supplementation, regardless of their T-score.
In two one-year placebo-controlled clinical trials, risedronate in a dosage of 5 mg per day was effective for the prevention34 and treatment35 of glucocorticoid-induced osteoporosis. An analysis36 of data from the two trials demonstrated a 70 percent reduction (P = .01) in the risk of vertebral fractures in the patients treated with risedronate.
In a study37 of 477 men and women receiving glucocorticoid therapy, alendronate in a dosage of 5 or 10 mg per day for 48 weeks increased BMD but did not significantly reduce the risk of vertebral fractures (morphometrically defined) compared with placebo. The primary outcome was a change in spinal BMD.
HORMONE THERAPY
Hormone therapy is approved for the prevention but not the treatment of postmeno-pausal osteoporosis. The Women’s Health Initiative (WHI) evaluated the effectiveness of hormone therapy in reducing the incidence of coronary heart disease and overall health risks in 16,608 predominantly healthy postmenopausal women. A subanalysis of WHI data38 indicated that hormone therapy reduced observed clinical vertebral fractures (relative risk [RR], 0.65; 95 percent confidence interval [CI], 0.46 to 0.92) and hip fractures (RR, 0.67; 95 percent CI, 0.47 to 0.96) by one third compared with placebo. However, hormone therapy was associated with increased risks of breast cancer, heart attacks, strokes, and blood clots in the lung; these risks appear to outweigh the benefits of hormone therapy for fracture prevention.38
RECOMBINANT HUMAN PARATHYROID HORMONE
Teriparatide (Forteo), a recombinant human parathyroid hormone (rhPTH[1-34]), is approved for the treatment of postmenopausal osteoporosis. In one placebo-controlled study,39 daily injections of rhPTH(1–34) reduced the risk of new vertebral fractures by 65 percent (20-mcg injections) and 69 percent (40-mcg injections; P ≤ .001), and the risk of new nonvertebral fractures by 35 percent (20-mcg injections) and 40 percent (40-mcg injections; P < .05). Because the long-term effects of teriparatide are not known, this agent is approved for a maximum of two years of use in patients with severe osteoporosis who are at high risk for fractures.
SELECTIVE ESTROGEN RECEPTOR MODULATORS
Raloxifene (Evista) is the only selective estrogen receptor modulator that has been approved for the prevention and treatment of postmenopausal osteoporosis. In the Multiple Outcomes of Raloxifene Evaluation (MORE) trial40 in women with postmenopausal osteoporosis, three years of raloxifene therapy in a dosage of 60 mg per day reduced the risk of vertebral fractures by 30 percent (CI, 0.6 to 0.9) in patients with vertebral fractures at baseline and by 50 percent (CI, 0.4 to 0.8) in patients without vertebral fractures at baseline. The MORE trial found no significant reduction in the risk of nonvertebral fractures.
SALMON CALCITONIN
Both intranasal and injectable forms of salmon calcitonin (Miacalcin) are approved for the treatment of postmenopausal osteoporosis. Calcitonin inhibits bone resorption and is recommended for use in women with osteoporosis who are at least five years past menopause and cannot take other agents. In the Prevent Recurrence of Osteoporotic Fractures (PROOF) trial,41 five years of calcitonin therapy in a dosage of 200 IU per day reduced the risk of new vertebral fractures by 33 percent (P = .03) in postmenopausal women with established osteoporosis. The PROOF study found no significant effect for calcitonin on hip BMD or risk of nonvertebral fractures.