
Am Fam Physician. 2004;70(11):2145-2152
Heart failure is a common, progressive, complex clinical syndrome with high morbidity and mortality. Coronary artery disease is its most common cause. The evaluation of symptomatic patients with suspected heart failure is directed at confirming the diagnosis, determining the cause, identifying concomitant illnesses, establishing the severity of heart failure, and guiding therapy. The initial evaluation should include a focused history and physical examination, a chest radiograph, and an electrocardiogram. The presence of heart failure can be confirmed by an echocardiogram. Heart failure is highly unlikely in the absence of dyspnea and an abnormal chest radiograph or electrocardiogram. Radionuclide angiography or contrast cineangiography may be necessary when clinical suspicion for heart failure is high and the echocardiogram is equivocal. Patients with confirmed heart failure should undergo additional testing, including a more detailed history and physical examination; a complete blood count; blood glucose measurement; liver function tests; serum electrolyte, blood urea nitrogen, and creatinine measurements; lipid panel; urinalysis; and thyroid-stimulating hormone level. A serum ferritin level, human immunodeficiency virus test, antinuclear antibody assays, rheumatoid factor test, or metanephrine measurements may be required in selected patients. Patients with coronary artery disease, hypertension, diabetes mellitus, exposure to cardiotoxic drugs, alcohol abuse, or a family history of cardiomyopathy are at high risk for heart failure and may benefit from routine screening.
Heart failure is characterized by an inability of the myocardium to deliver sufficient oxygenated blood to meet the needs of tissues and organs during exercise or at rest. Because diagnostic criteria for this clinical syndrome remain ill defined, the actual prevalence is difficult to determine. Heart failure is estimated to affect 2 to 4.5 million persons in the United States.1–3 The condition is more common in men than in women, and its prevalence increases with age (1.1 percent in persons 25 to 54 years of age, 3.7 percent in persons 55 to 64 years, and 4.5 percent in persons 65 to 74 years).3 Heart failure is becoming increasingly common as the U.S. population ages and survival rates after acute myocardial infarction increase.
| Key clinical recommendation | Levels | References |
|---|---|---|
| Screening the general population for heart failure is not recommended, but screening high-risk patients may be appropriate. | C | 8 |
| The initial evaluation of patients with suspected heart failure should include a focused history and physical examination, an ECG, and a chest radiograph. An echocardiogram can confirm the diagnosis. | C | 8 |
| Dependent edema and pulmonary rales are of limited value in diagnosing heart failure resulting from left ventricular dysfunction. | B | 12,14 |
| Heart failure can be ruled in if jugular venous distention, displacement of the apical pulsation, or a gallop rhythm is present. | B | 12 |
| Absence of dyspnea or a normal ECG and chest radiograph make the diagnosis of heart failure highly unlikely. | B | 12,13,14 |
| If heart failure is confirmed by an echocardiogram, a more detailed history and physical examination, a complete blood count, blood glucose level, liver function tests, serum electrolyte levels, serum lipid panel, blood urea nitrogen level, creatinine level, urinalysis, and thyroid-stimulating hormone level should be obtained. | C | 8 |
The annual direct medical cost of caring for patients with heart failure is estimated to exceed $10 billion.4 Furthermore, heart failure is a progressive condition: once symptoms appear, subsequent morbidity and mortality are high. In patients with heart failure identified by careful screening, five-year survival rates are only 59 percent in men and 45 percent in women.5
This article focuses on the diagnosis of heart failure from an evidence-based perspective. A clinical review6 published in this issue examines the treatment of heart failure and the prognosis for affected patients.
Pathophysiology of Heart Failure
Normal myocardial function requires sufficient nutrient-rich, toxin-free blood at rest and during exercise; sequential depolarization of the myocardium; normal myocardial contractility during systole and relaxation during diastole; normal intracardiac volume before contraction (preload); and limited resistance to the flow of blood out of the heart (afterload). The capacity of the heart to adapt to short-term changes in preload or afterload is remarkable, but sudden or sustained changes in preload (e.g., acute mitral regurgitation, excessive intravenous hydration), afterload (e.g., aortic stenosis, severe uncontrolled hypertension), or demand (e.g., increased demand because of severe anemia or hyperthyroidism) may lead to progressive failure of myocardial function. Asymptomatic dysfunction progresses steadily to overt heart failure.
Coronary artery disease accounts for nearly 70 percent of all cases of heart failure.7 Less frequent causes include diabetes mellitus and valvular heart disease (Table 1). Heart failure also can be multifactorial. For example, the disease can result from acute myocardial infarction (loss of myocardial contractility) with papillary muscle dysfunction (increased preload) and acute pulmonary edema (hypoxemia).
| Common |
| Coronary artery disease |
| Hypertension |
| Idiopathic |
| Less common |
| Diabetes mellitus |
| Valvular disease |
| Rare |
| Anemia |
| Connective tissue disease |
| Viral myocarditis |
| Hemochromatosis |
| Human immunodeficiency virus infection |
| Hyperthyroidism, hypothyroidism |
| Hypertrophic cardiomyopathy |
| Infiltrative disease (including amyloidosis and sarcoidosis) |
| Mediastinal radiation |
| Peripartum cardiomyopathy |
| Restrictive pericardial disease |
| Tachyarrhythmia |
| Toxins (including drugs and alcohol) |
| Trypanosomiasis (Chagas’ disease) |
Heart failure may be classified into six types based on the role of diastolic or systolic dysfunction (Table 2). Diastolic dysfunction is heart failure caused by compromised myocardial relaxation in the presence of normal myocardial contractility and ejection fraction. It is associated most commonly with coronary artery disease, hypertension, aging, and infiltrative cardiomyopathy. Systolic dysfunction is caused by impaired myocardial contractility and low ejection fraction. It is associated most often with coronary artery disease (especially myocardial infarction), idiopathic dilated cardiomyopathy, hypertension, and valvular disease.
| Type | Characteristics | |
|---|---|---|
| Diastolic dysfunction | Normal myocardial contractility, left ventricular volume, and ejection fraction; impaired myocardial relaxation; diminished early diastolic filling | |
| Systolic dysfunction | Absolute or relative impairment of myocardial contractility, low ejection fraction | |
| High output heart failure | Bounding pulses, wide pulse pressure, accentuated heart sounds, peripheral vasodilatation, increased cardiac output and ejection fraction, moderate four-chamber enlargement | |
| Low cardiac output syndrome | Fatigue, loss of lean body mass, prerenal azotemia, peripheral vasoconstriction, reduced left or right contractility | |
| Right heart failure | Dependent edema, jugular venous distention, right atrial and ventricular dilatation, reduced right-sided contractility | |
| Left heart failure | Dyspnea, pulmonary vascular congestion, reduced left-sided contractility | |
| Biventricular failure | Dyspnea, dependent edema, jugular venous distention, pulmonary vascular congestion, bilateral reduced contractility | |
The five types of heart failure resulting from systolic dysfunction include high output heart failure, low cardiac output syndrome, right heart failure, left heart failure, and biventricular failure. High output heart failure occurs when the demand for blood exceeds the capacity of an otherwise normal heart to meet the demand. This type of heart failure may occur in patients with severe anemia, arteriovenous malformations with shunting of blood, or hyperthyroidism. Patients with low cardiac output syndrome have fatigue and loss of lean muscle mass as their most prominent symptoms, but they also may have dyspnea, impaired renal function, or altered mental status. Right heart failure is characterized by peripheral edema, whereas left heart failure is characterized by pulmonary congestion. Both systemic and pulmonary congestion are present in patients with biventricular heart failure.
Although the symptoms, causes, prevalence, and epidemiology of the six different types of heart failure are somewhat different, there is substantial overlap, and types may coexist. Therefore, this review presents an approach to diagnosis that is appropriate regardless of the type or cause of heart failure.
Overview of Diagnosis
The spectrum of patients who may be suspected of having heart failure ranges from those who are asymptomatic but at high risk for heart failure (i.e., patients who abuse alcohol or have coronary artery disease, hypertension, diabetes mellitus, exposure to cardiotoxic drugs, or familial history of cardiomyopathy) to those with florid signs and symptoms of heart failure.
Guidelines from the American College of Cardiology and the American Heart Association8 identify four stages in the progression of heart failure. Patients in stage A have no structural abnormalities but are at high risk for heart failure. In stage B, patients are asymptomatic but have structural heart disease. Patients in stage C have structural abnormalities and past or present heart failure. In stage D, patients have end-stage heart failure and require mechanical circulatory support, infusion of inotropic agents, cardiac transplantation, or hospice care.
The presence of asymptomatic patients, the progressive nature of heart failure, the high morbidity and mortality rates associated with the condition, and the fact that early treatment can delay the onset of overt heart failure have caused some investigators to speculate about the need to screen patients for heart failure.9 Screening of the general population currently cannot be recommended.10 However, screening echocardiography may be appropriate in selected patients who are at high risk for developing systolic dysfunction, such as patients with a strong family history of cardiomyopathy and patients with exposure to cardiotoxic drugs.8
The evaluation of symptomatic patients with suspected heart failure is directed at confirming the presence of heart failure, determining the cause, identifying comorbid illnesses, establishing the severity of heart failure, and guiding therapy. The first four purposes of the evaluation are discussed in this article. Therapy is reviewed in another article.6
Confirming the Presence of Heart Failure
Heart failure is a clinical diagnosis, and no single test can establish its presence or absence. In patients with this condition, the most frequent clinical findings are related to decreased exercise tolerance or fluid retention11 (Table 3).12–15 Decreased exercise tolerance typically presents as dyspnea or, much less commonly, fatigue on exertion. Fluid retention results in orthopnea, rales, elevated jugular venous pressure, dependent edema, and the typical radiographic findings of cardiomegaly, pulmonary edema, and pleural effusion. Unfortunately, there currently are no validated clinical decision rules to estimate the contribution of each of these findings to heart failure.
Nearly all patients with heart failure present with dyspnea. The absence of dyspnea makes heart failure highly unlikely (sensitivity: greater than 95 percent), and other explanations for the patient’s symptoms should be sought first.
It is important to note that heart failure is present in only about 30 percent of patients who present with dyspnea in the primary care setting.16 Other common causes of dyspnea in adult primary care patients include asthma (33 percent), chronic obstructive pulmonary disease (9 percent), arrhythmia (7 percent), infection (5 percent), interstitial lung disease (4 percent), anemia (2 percent), and pulmonary embolism (less than 2 percent).16 Therefore, 30 percent is a reasonable pretest estimate of the probability of systolic or diastolic heart failure in patients presenting with dyspnea in the primary care setting.
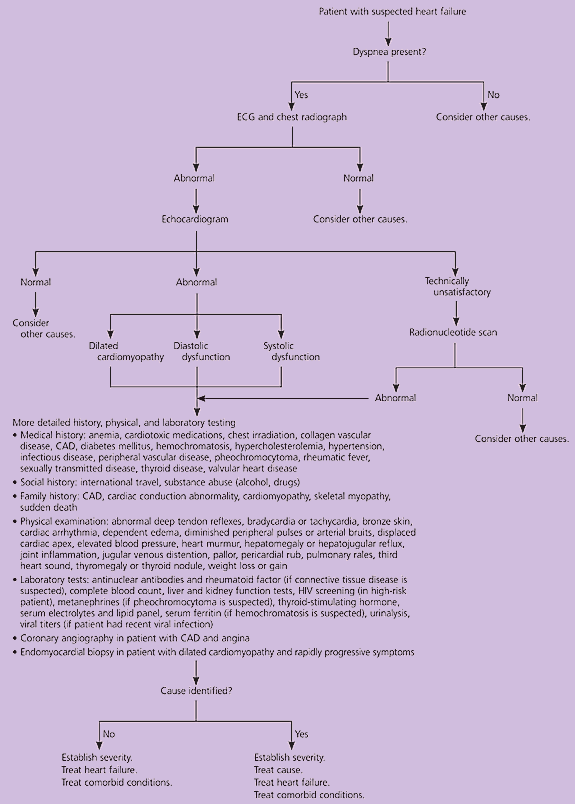
In patients with dyspnea, a focused history and physical examination, combined with selected diagnostic testing, can identify heart failure (Figure 1).8,12–14 This diagnostic approach, which avoids unnecessary testing and expense, is guided by the sensitivity and specificity (or likelihood ratios) of various clinical findings12–14,17 (Table 3).12–15
| Clinical finding | Reference standard | Sensitivity (%) | Specificity (%) | Positive likelihood ratio (95 percent CI) | Negative likelihood ratio (95 percent CI) | |
|---|---|---|---|---|---|---|
| History | ||||||
| Dyspnea on exertion | LV dysfunction on echocardiogram | 100 | 17 | 1.2 (1.1 to 1.3) | 0 (0 to 0.1) | |
| Paroxysmal nocturnal dyspnea | LV dysfunction on echocardiogram | 39 | 80 | 2.0 (1.2 to 3.1) | 0.8 (0.6 to 1.0) | |
| Previous myocardial infarction | LV dysfunction on echocardiogram | 59 | 86 | 4.1 (2.7 to 6.2) | 0.5 (0.3 to 0.7) | |
| Physical examination | ||||||
| Displaced cardiac apex | LV dysfunction on echocardiogram | 66 | 95 | 16.0 (8.1 to 31.0) | 0.4 (0.2 to 0.6) | |
| Dependent edema | LV dysfunction on echocardiogram | 20 | 86 | 1.4 (0.7 to 2.9) | 0.9 (0.8 to 1.1) | |
| Gallop rhythm | LV dysfunction on echocardiogram | 24 | 99 | 27.0 (6.1 to 120.0) | 0.8 (0.6 to 0.9) | |
| Hepatojugular reflux | Clinicoradiographic score | 33 | 94 | 6.0 (1.3 to 29.0) | 0.7 (0.5 to 1.1) | |
| Jugular venous distention | LV dysfunction on echocardiogram | 17 | 98 | 9.3 (2.9 to 30.0) | 0.8 (0.7 to 1.0) | |
| Pulmonary rales | LV dysfunction on echocardiogram | 29 | 77 | 1.3 (0.7 to 2.2) | 0.9 (0.7 to 1.1) | |
| Tests | ||||||
| Chest radiograph: cardiomegaly, pulmonary edema, or both | LV dysfunction on echocardiogram | 71 | 92 | 8.9 (2.1 to 83.0) | 0.3 (0.2 to 0.6) | |
| ECG: anterior Q waves or LBBB | LV dysfunction on echocardiogram | 94 | 61 | 2.4 (2.1 to 2.8) | 0.1 (0 to 0.2) | |
| BNP level (pg per mL) | ||||||
| ≥ 150 | Blinded clinical assessment by two cardiologists | — | — | 5.0 (4.4 to 5.5) | — | |
| 100 to 149 | As above | — | — | 0.7 (0.6 to 1.0) | — | |
| 50 to 99 | As above | — | — | 0.5 (0.4 to 0.6) | — | |
| < 50 | As above | — | — | 0.05 (0.03 to 0.06) | — | |
A history of myocardial infarction is of limited assistance in the diagnosis of heart failure. A positive history only slightly increases the probability of heart failure, and a negative history is associated with only a small decrease in probability. Likewise, dependent edema provides minimal help in diagnosing heart failure. If present, hepatojugular reflux increases the likelihood of heart failure moderately; absence of this finding does little to reduce the likelihood of heart failure.12,17 Heart failure can be ruled in if jugular venous distention, displacement of cardiac apical pulsation, or a gallop rhythm is present (specificity: 95 percent or greater); however, absence of these findings is of limited help in ruling out heart failure. It is important to note that the ability to detect physical findings of heart failure depends on proper technique and the skill of the examiner (Table 4).17
| Physical finding | Technique |
|---|---|
| Abdominojugular reflux | Patient position: supine, so that the top of the jugular venous pulsation is seen in the right side of the neck |
| Encourage the patient to relax and breathe normally. apply firm steady pressure (25 to 30 mm Hg) to the midabdomen for 30 seconds. The test is positive if there is a sustained (≥ 10-second) 4-cm rise in the venous pressure. | |
| Displaced cardiac apex | Patient position: supine or 45-degree-angle left lateral decubitus |
| Palpate the fourth and fifth left intercostal space during expiration. The test is positive if the impulse is outside the midclavicular line. | |
| Gallop rhythm | Patient position: 45-degree-angle left lateral decubitus |
| Listen with the bell of the stethoscope lightly applied to the chest wall. | |
| Jugular venous distention | Patient position: supine at 45-degree angle, with head turned to the right |
| Perform this test in a well-lit room. adjust the incline of the bed until the top of venous pulsation is visible above the angle of the jaw. measure the distance to the level of the angle of louis. |
A chest radiograph and an electrocardiogram should be obtained in patients with dyspnea and suspected heart failure. A normal chest radiograph slightly decreases the probability of heart failure and helps identify pulmonary causes of dyspnea. A normal electrocardiogram makes heart failure unlikely (sensitivity: 94 percent). If both the electrocardiogram and chest radiograph are normal, heart failure is highly unlikely (sensitivity: 95 percent or greater), and other causes should be considered.13,14
Heart failure is strongly suggested by the presence of cardiomegaly or pulmonary vascular congestion on the chest radiograph. The probability of heart failure is increased by anterior Q waves or left bundle branch block on the electrocardiogram. Therefore, patients with dyspnea and suggestive abnormalities on the electrocardiogram or chest radiograph should undergo two-dimensional echocardiography with Doppler flow studies. The echocardiogram is the diagnostic standard for identifying both systolic and diastolic heart failure. Radionuclide angiography or contrast cineangiography may be helpful if the echocardiogram is equivocal or technically inadequate.18,19
If the B-type natriuretic peptide (BNP) level is extremely low (less than 50 pg per mL), heart failure is highly unlikely. Conversely, a BNP level of 150 pg per mL or greater is moderately helpful (specificity: 83 percent) in ruling in heart failure15 (Table 3).12–15 However, the independent contribution of BNP to the diagnosis of heart failure has not been determined, and further studies are required to delineate the role that this peptide should play in the diagnosis of heart failure.
The diagnosis of diastolic dysfunction is problematic. Diagnostic criteria for this type of heart failure are poorly defined, diastolic dysfunction often is present in patients who also have left ventricular systolic dysfunction, and most patients with diastolic dysfunction have other conditions that could explain their symptoms.20,21 Currently, Doppler echocardiography is the primary tool for identifying abnormal diastolic function, including diminished early diastolic filling and reduced ventricular compliance associated with diastolic dysfunction.22
Identifying Causes and Comorbidities of Heart Failure
Individually or in combination, myocardial, valvular, pericardial, and systemic diseases may cause heart failure (Table 1). As previously noted, heart failure can result from increased demand, systolic dysfunction, or diastolic dysfunction. Heart failure with normal left ventricular systolic function must be distinguished from respiratory disease, obesity, and myocardial ischemia.20
The history, physical examination, and laboratory evaluation may provide clues to the type of heart failure, its cause, and any comorbidities (Table 5). The Doppler echocardiogram can identify systolic and diastolic dysfunction, and it may identify valvular stenosis or insufficiency, cardiomyopathy, or pericardial disease.
| Clinical finding | Implication | |
|---|---|---|
| History | ||
| Fatigue | Low cardiac output syndrome | |
| Nausea or abdominal pain | Hepatic congestion resulting from right ventricular dysfunction | |
| Alcohol use, anemia, cardiotoxic medications, chest irradiation, connective tissue disease, exposure to cardiotoxic medications, exposure to sexually transmitted disease (e.g., human immunodeficiency virus infection), hemochromatosis, hyperthyroidism, hypothyroidism, infectious diseases, pheochromocytoma | Cardiomyopathy | |
| Chest irradiation, connective tissue disease, previous open heart surgery | Restrictive pericarditis | |
| Coronary artery disease, diabetes mellitus, hypercholesterolemia, hypertension, peripheral vascular disease, tobacco use | Coronary artery disease | |
| Physical examination | ||
| Abnormal deep tendon reflexes, bradycardia or tachycardia, bronze skin, joint inflammation, pallor, thyromegaly or thyroid nodule | Cardiomyopathy | |
| Ascites, dependent edema, hepatomegaly, hepatojugular reflux, jugular venous distention, weight gain | Right ventricular dysfunction | |
| Cool extremities, cyanosis, weight loss | Low cardiac output syndrome | |
| Diminished peripheral pulses or arterial bruits | Coronary artery disease | |
| Displaced cardiac apex, pulmonary rales, pulse rate higher than 90 beats per minute, systolic blood pressure below 90 mm Hg, third heart sound | Left ventricular dysfunction | |
| Heart murmur | Valvular heart disease | |
| Laboratory tests | ||
| Anemia, abnormal thyroid-stimulating hormone level | Cardiomyopathy | |
| Elevated blood urea nitrogen and creatinine levels | Low cardiac output syndrome | |
| Elevated liver function values | Hepatic congestion resulting from right ventricular dysfunction | |
| Hyperglycemia, hyperlipidemia | Coronary artery disease | |
Even if the echocardiogram identifies the cause of heart failure, a broad spectrum of illnesses may exacerbate the condition. Therefore, the initial evaluation of patients with confirmed heart failure must identify concomitant illnesses as well as the primary cause (Figure 1).8,12–14 This evaluation also may identify patients who require additional testing, such as a serum ferritin measurement, viral titers, a human immunodeficiency virus test, antinuclear antibody assays, a rheumatoid factor test, or metanephrine measurements.8 Rarely, patients may require coronary angiography or endomyocardial biopsy.8
Establishing the Severity of Heart Failure
The severity of heart failure at the time of initial diagnosis is helpful in determining prognosis, monitoring disease progression, and evaluating response to treatment.
In symptomatic patients, the level of exertion required to cause symptoms reflects the degree of myocardial impairment, but it is important to recognize that the correlation between cardiac function and symptoms is not strong. Nevertheless, symptoms are the basis of the New York Heart Association (NYHA) classification of heart failure, which often is used to determine prognosis.23 In NYHA class I heart failure, symptoms occur with greater than ordinary physical activity. Patients with NYHA class II heart failure have symptoms with ordinary physical activity. In NYHA class III heart failure, symptoms occur with minimal physical activity. Patients with NYHA class IV heart failure have symptoms while at rest.
The ejection fraction (as measured by the echocardiogram) and the six-minute walk test independently predict mortality in patients with left ventricular dysfunction. The six-minute walk test is performed by having the patient walk a 30.48-m (100-ft) course 15.24 m (50 ft) in each direction in a hall, with a chair positioned at each end of the course) for six minutes. The patient is allowed to stop and rest as often as desired but is encouraged to continue walking. After six minutes, the total distance walked is measured and recorded to the nearest meter or foot. The distance walked correlates well with subsequent hospitalization and death (Table 6).24 This simple test also may be helpful in monitoring disease progression and response to treatment.
In routine clinical settings, the 35 percent five-year mortality rate among all patients with newly diagnosed heart failure is about 50 percent higher in patients with NYHA class III or IV heart failure.11 The one-year mortality rate increases by about 75 percent for every 15 percent drop in ejection fraction and by about 50 percent for each 120-m (394-ft) decrease in the distance walked on the six-minute walk test.